|
Cells taken from the nose may have great potential
A woman in the US has developed a tumor-like growth eight years after a stem cell treatment to cure her paralysis failed.
There have been a handful of cases of stem cell treatments causing growths but this appears to be the first in which the treatment was given at a Western hospital as part of an approved clinical trial.
At a hospital in Portugal, the unnamed woman, a US citizen, had tissue containing olfactory stem cells taken from her nose and implanted in her spine. The hope was that these cells would develop into neural cells and help repair the nerve damage to the woman's spine. The treatment did not work - far from it. Last year the woman, then 28, underwent surgery because of worsening pain at the implant site.
The surgeons removed a 3-centimetre-long growth, which was found to be mainly nasal tissue, as well as bits of bone and tiny nerve branches that had not connected with the spinal nerves.
The growth wasn't cancerous, but it was secreting a "thick copious mucus-like material", which is probably why it was pressing painfully on her spine, says Brian Dlouhy at the University of Iowa Hospitals and Clinics in Iowa City, the neurosurgeon who removed the growth.
The results of the surgery have now been published.
Unpredictable consequences
The case shows that even when carried out at mainstream hospitals, experimental stem cell therapies can have unpredictable consequences, says Alexey Bersenev, a stem cell research analyst who blogs at Cell Trials.
Stem cells have the prized ability to divide and replenish themselves, as well as turn into different types of tissues.
There are several different stem cells, including ones obtained from an early embryo, aborted fetuses, and umbilical cord blood. There are many sources within adult tissues, too, including bone marrow.
While often hailed as the future of medicine, stem cells' ability to proliferate carries an inherent danger and the fear has always been that when implanted into a person they could turn cancerous.
Lawsuits abound
Still, a few stem cell therapies have now been approved, such as a treatment available in India that takes stem cells from the patient's eye in order to regrow the surface of their cornea, and a US product based on other people's bone stem cells.
Many groups around the world are investigating a wide range of other applications, including treating,
Research groups at universities and hospitals need to meet strict safety guidelines for clinical trials but some small private clinics are offering therapies to people without research or marketing approval.
There is a growing number of lawsuits against such clinics and a few cases have been reported of tumors or excessive tissue growth (see "Ongoing stem cell trials" below).
When the Italian government tried to ban an unproven stem cell therapy for neurological disorders, the treatment's supporters - families of people receiving the therapy who thought it was working - protested in the street.
Following the nose
The woman at the centre of today's news was treated at the Hospital de Egas Moniz in Lisbon, where a team got approval for early-stage trials to explore the potential of nasal cells in treating paralysis.
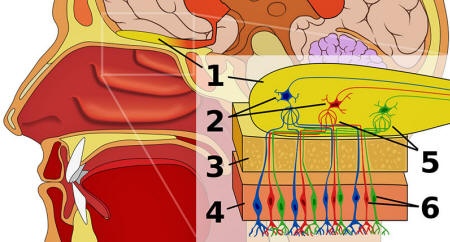
Tissue at the top of the nasal passages contains both olfactory stem cells and "olfactory ensheathing cells", which support and guide the growth of the neurons.
Your olfactory system. 1 is the olfactory bulb (the bit of your brain that processes smells); 6 is the olfactory receptors that bind to specific chemicals (odors). [Image credit: Wikipedia]
Other groups are experimenting with taking such tissue from the nose, growing it in the lab to isolate the desired cells and transplanting them.
But the Lisbon clinic was trialing a procedure that bypassed this isolation step. They took small pieces of the nasal lining and directly transplanted them into their patients' spines.
That could be why it retained its capacity to make mucus, says Dlouhy.
In 2010, the Lisbon researchers published their results using this method on 20 people paralysed at various locations in their spine. Eleven experienced some recovery of movement or sensation; one person's paralysis got worse, one developed meningitis and four others experienced minor adverse events.
It is not clear whether the woman from the US was part of this trial.
Extreme vigilance
New Scientist was unable to reach the Lisbon team members, but Jean Peduzzi-Nelson, a stem cell researcher at Wayne State University in Detroit, Michigan, who advised the team on their surgical technique - she had previously tested it on rodents - claims the clinic has given the therapy to about 140 people in total.
Peduzzi-Nelson adds that most of the recipients of the nasal tissue who received the right kind of rehabilitation after their surgery experienced improvement.
But the case shows that even patients who feel they have nothing to lose should be cautious, says Leigh Turner of the University of Minnesota in Minneapolis, who tracks lawsuits involving stem cell therapies.
Daley points out that many trials track their patients for only a few years, so could miss such delayed problems.
Journal reference
|