|

by Elysium Health
August
16, 2018
from
EndPoints website
|
Endpoints is a science publication by Elysium Health, a
consumer health company translating advances in science
and technology into effective, scientifically-sound
health products.
All
stories on this site are meant for educational purposes
- to encourage scientific literacy and improve the
public perception of science. |

Illustrations by Kevin Tong
It's been
called the "forgotten organ,"
a "scientific
frontier," and even the "second brain,"
yet scientists
say we have barely scratched the surface
on understanding
the role of this living, breathing,
highly complex
ecosystem in shaping human health.
Here's an
up-close look at what we know so far...
Humans have a complicated, you might even say, fraught, relationship
with bacteria.
It's everywhere. Around us. On us. Within us...
Yet,
for centuries we've drawn a strong causal link between bacteria and
illness - and for good reason. Devastating outbreaks and epidemics
from the bubonic plague and rheumatic fever to whooping cough and
anthrax, and even some of today's most perplexing illnesses, Lyme
disease, MRSA, and others, can all be traced back to these
remarkably intelligent and highly adaptive microorganisms.
But just over the past two decades, scientists have begun to shift
that paradigm, poking holes in the dogma that all bacteria are
'dangerous' and to be avoided at all costs, or whenever possible,
power washed away.
Today, there's mounting evidence implicating the
10,000 or so different types of,
-
bacteria
-
archaea
-
fungi
-
viruses
-
protozoa
-
helminths,
...inhabiting all of us, known collectively
along with their genetic material as
the microbiome, as crucial to
our survival, influencing every aspect of health from mood to
weight.
"We have a long, shared history with the microbiome," says
Martin J. Blaser, M.D., a professor of microbiology and director of
the Human Microbiome Program at the New York University School of
Medicine.
Martin J. Blaser's lab studies the biology of bacterial colonization
with a lens on the interactions that lead to or protect from us
disease.
"It is intimately involved in human biology and so broad in its
possible implications."
In this first installment of a two-part series, we introduce the
microbiome and highlight some of the most important research about
its associations with human health and disease.
"We have an
integrated circuit with our microbiology, and therefore if we want
to understand human health, we have to understand the microbiome and
we have to understand its relationships," says Blaser.
Before You
Start - Key Terms to Understand
-
Diversity:
A
measure of how many different species and, dependent on the
diversity indices, how evenly distributed they are in a microbiome. Lower diversity is considered a marker of
dysbiosis (microbial imbalance) in the gut and has been
found in autoimmune diseases and obesity and cardiometabolic
conditions, as well as in older adults.
-
Dysbiosis:
A
disturbance or imbalance in the microbial communities either
in or on the body that can be caused by factors such as
diet, stress,
antibiotics, oral contraceptive pills, and
lifestyle. Dysbiosis has been associated with health
problems, including inflammatory bowel disease and chronic
fatigue syndrome.
-
Enterotype:
A
collection of species of bacteria in the gut microbiome that
are found to be influenced by diet. Scientists have
identified three human enterotypes,
-
Bacteroides
-
Prevotella
-
Ruminococcus,
...but there's still much debate about their
importance to health and disease and whether distinct
boundaries between the three groupings even exist.
-
Flora:
The name
previously given to the bacterial communities inhabiting our
gastrointestinal tract. Researchers now prefer the term gut microbiota.
-
Metabolome:
The
collection of metabolites, byproducts made or used when the
body breaks down food, drugs, chemicals, or its own tissue
(i.e., glucose and fatty acids), found within an organism,
cell, or tissue. In metabolomics, researchers study the
metabolome to understand the relationship between the
microbiome and the body's life-sustaining chemical
reactions.
-
Metagenome:
The
collection of genomes and genes from the organisms in a microbiota. Metagenomics is the field of molecular research
that studies the complexity of microbiomes.
-
Metatranscriptome:
A collection of
messenger RNA molecules expressed from the
genes of organisms in a microbiota.
Metatranscriptomics is a
powerful RNA sequencing technology that allow analysis of
complex microbial communities and their gene expression and
regulation.
-
Pathogen:
An
infectious biological agent that can produce a disease in
its host.
-
Proteome:
A
complete set of proteins expressed by an organism. The study
of the proteome is called
proteomics, and it involves
understanding how proteins function and interact with one
another. Metaproteomics refers to the large-scale
characterization of the entire protein complement of
environmental or clinical samples at a given point in time.
-
Phenotypes:
Observable physical traits (i.e., appearance, development,
and behavior) of an organism determined by its
genotype,
which is the set of genes the organism carries, as well as
by environmental influences upon these genes.
-
Short chain fatty
acids:
Fatty acids with two to six carbon atoms that are
produced by bacterial fermentation of dietary fibers in the
gut. These acids have been shown to play an important role
in regulating metabolism; low levels of
SCFAs are associated
with gastrointestinal disorders and obesity.
What Exactly
Is the Microbiome?
You've probably heard the terms "microbiota" and "microbiome" used
interchangeably, but there's an important distinction.
Microbiota is
the dynamic community of trillions of microbes - short for
microscopic organisms - living in harmony with your human (eukaryotic)
cells. Your microbiome, on the other hand, is the collective name
given to the genes inside these microbes.
This genetic material is
essentially what scientists are studying in hopes of uncovering the
truth behind how and why microbes are involved in health.
The number of genes in all the microbes in one person's microbiome
is
approximately 150 times the number of genes in the human genome.
Noted molecular biologist and Nobel laureate Joshua Lederberg
described it in 2001, when the study of the microbiome was in its
infancy as,
"the ecological community of commensal, symbiotic, and
pathogenic microorganisms that literally share our body space and
have been all but ignored as determinants of health and disease."
Almost two decades later, study after study has shown that our
microbiome plays an integral role in boosting immunity, preventing
infection, and keeping our digestive system running smoothly, our
hormone levels balanced, and our brains working properly.
Our microbiome may even predict our risk for developing certain chronic
diseases.
A large-scale study (Environment
Dominates over Host Genetics in shaping Human Gut Microbiota) published in the journal
Nature in
February revealed a strong association between the microbiome data
of more than 1,000 healthy adults and measurements of cholesterol,
weight, blood glucose levels, and other clinical parameters.
The
study's authors concluded that using human genetic data together
with microbiome profiles significantly improved how accurately they
could predict the subjects' metabolic traits, compared to using
genetic data alone.
How Much of Us Is Microbes?
Although various numbers of been reported dating back to the early
1970s, from 100 billion to hundreds of trillions, a
2016 PLOS One
analysis weighing the available evidence put the estimate at around
39 trillion microbial cells and 30 trillion human cells for the
average adult, with variations based on weight, height, sex, and
age.
But because of their small size, microorganisms make up about
one to three percent of the body's mass.
All told, they weigh
between two and six pounds, as much as or even more than your brain.
The placental microbiome
has a taxonomic profile
that is similar to
the oral microbiome.
Strong phylum-level similarity
was observed
between the placenta
and tongue, tonsils, saliva, and
subgingival
plaque taxonomic profiles.
The colors of dots reflect the vicinity
of the body sites.
Sci Transl Med. 2014 May 21
6(237): 237ra65.
Where Is Your
Microbiome?
Microbes exist everywhere on and in the human body, although there
are specific areas of the body that contain large concentrations of
microbes.
The vast majority of our microbes reside in the
gastrointestinal tract, known as the gut microbiota (formerly called
the gut flora), it
harbors up to 1,000 species of microbes.
Gut microbiomes from different people can contain similar species of
microbes, but vary by strain from one person to the next. Aside from
the gut, other most-studied sites of the microbiome include the
eyes, lungs, mouth, nasal cavity, skin, and vagina.
During early childhood, the composition of your microbiome changes
frequently, but by around age three, it becomes fairly stable. Stable, but not static.
Your microbiome remains malleable throughout
your entire life...
While the triggers for the ongoing microbial
changes aren't fully understood, a number of factors from what you
eat to where you live and your age, race, sex, hormonal cycles, and
even the medicines have all been implicated.
For example, studies
show that puberty triggers changes in your skin microbiome and the
composition and structure of the vaginal microbiome shifts during
and after pregnancy, and then again during menopause.
Our microbiomes can even be found outside our bodies, on nearly
every surface and environment we come in contact with.
That's
because just by the simple act of entering a room, you're shedding
microbes into the air, referred to by scientists as your
microbial
cloud.
When Do You
Get Your First Microbes?
For most of us, our introduction to microbiota begins during birth.
Infants are exposed to the microbial population of the birth canal
on arrival into the world, which influences the development of their
gut microbiota.
Infants delivered through C-section show reduced
numbers of gut microbes compared to those delivered vaginally,
however, studies show that difference is less detectable by six
months.
More recent research, within the last decade or so, suggests that we
may be exposed to microbes in utero, calling into question the
long-held belief by the medical community that the womb is a
pristine, sterile environment...
A 2013 study of placentas taken from 195 patients conducted by
researchers at the Washington University School of Medicine and
published in 2013 found bacteria present in nearly a third of
placentas. (The placenta carries oxygen, nutrients, and more from
mother to infant, and also provides a defense system against
infections.)
A larger study (The
Placenta Harbors a Unique Microbiome) published a year later by researchers at
Baylor College of Medicine using gene sequencing tested placenta
specimens from more than 300 patients.
They found bacteria in the
placentas of many, including healthy pregnancies, suggesting that
the bacteria were an important part of development. The study also
showed that the bacteria in the placenta closely resembled that of
the oral microbiome.
Dozens of labs have replicated these findings,
and some have even detected microbes in amniotic fluid.

(Above left) A subset of healthy, vaginally born,
exclusively
breastfed infants (n = 42)
was used to train a random forest
regression model
that was then applied to estimate microbiota
maturity.
(Above right) Differences in relative microbiota maturity
based on the age at which solid food
was introduced in our cohort.
JAMA Pediatr. 2017;171(7):647–654.
doi:10.1001/jamapediatrics.2017.0378
The debate continues, but if fetal microbiomes do exist, that could
have far-reaching implications not only for medicine, but also for
basic biology.
For example, scientists might be able to find ways to
shift the microbial composition in the womb and possibly ward off
diseases.
Another early influence on your microbiome? Breast feeding...
One
study published last year in JAMA Pediatrics found that nearly 30
percent of infants derived their gut bacteria from microbes in their
mother's breast milk.
What Does the
Microbiome Have to Do With Health?
Most of the bacteria and other microbes that make up the microbiome
are actually beneficial to our health and carry out specific and
vital functions, such as,
"The microbiome makes
tons of metabolites and a good example is
vitamin B12, which is a
very complicated molecule," says
Michael Snyder, Ph.D., a genetics
professor and chair of the Department of Genetics at Stanford
University School of Medicine and a principal investigator of the Integrative Human Microbiome Project (iHMP).
"You don't make B12 for
yourself; in most cases, your microbes make it for you."

At the same time, autoimmune disorders and chronic diseases such as
rheumatoid arthritis, inflammatory bowel disease, metabolic
syndrome, which is closely linked to obesity and increases your risk
of diabetes and heart problems, and many others have been associated
with microbial dysfunction, or
dysbiosis.
Here's a summary of the
current knowledge on three broad pathways of human physiology.
A word of caution:
Experts, including Snyder and Blaser, advise
careful interpretation of studies on the microbiome, as the bulk of
the research has been carried out in mice and warrants further
investigation. In other words, correlation has yet to equal
causation.
"It's early days and there's a lot of hype out there,"
says Blaser.
"We have to do solid scientific work to figure out what's important
and what's not, and what's causal and how we can harness the
microbiome to improve health."
Digestion and
Nutrition
The microbiota is a key influence on digestion and probably the most
well-understood area in the study of the human microbiome, says
Snyder.
Without our gut microbes, many foods we eat, for example
plant cellulose, found in fruits, vegetables, nuts, would be
indigestible.
The human gastrointestinal tract is well equipped to break down
monosaccharides, such as glucose, and disaccharides, such as
lactose. But it has a much more difficult time digesting complex
molecules, or polysaccharides, such carbohydrates, lipids, and
proteins derived from meats and vegetables.
That's where gut
microbes come in.
Gut microbes feed off these molecules, breaking
them down through fermentation into byproducts called short chain
fatty acids - nutrients shown to be integral to energy metabolism
and appetite regulation - that can be absorbed and utilized by the
body.
For centuries, weight was tied to how much you eat, but newer
research has identified correlations between how much you weigh and
your microbes.
While some study results have captured the public's
attention - for, example, this one showing that mice who received a
"gut bacteria transplant" from an obese human gained more weight
than those who received bacteria from a lean human - researchers
have yet to arrive at a direct causal explanation, and studies have
produced varied results.
Some studies, for example, show that
microbes may use their metabolic activities to influence food
cravings and feelings of being full.
Emerging research in mice has
shown that a higher production of short chain fatty acids is
associated with a lower risk for obesity. And a recent study in
humans published in the Mayo Clinic Proceedings found that an
increased ability to metabolize carbohydrates may actually hinder
weight loss.
The study was small, and the authors caution that
further research is needed to validate the results.
Immunity and
Inflammation
Balance of your gut microbes highly influences the balance of your
immune system, says Snyder.
"You have more immune cells in your gut
than anywhere else, so your immune system and your microbiome are
always talking to each other."
Studies show that disruption in the
communication between the immune system and the gut microbiota can
throw off that balance, opening the door for pathogens that can
shift the immune system and may
contribute to complex diseases,
including,
-
allergies
-
obesity
-
diabetes
-
depression
-
even cancer...
Studies on mice raised in
gnotobiotic environments, unexposed to
both beneficial and pathogenic microorganisms, have helped to
provide extensive insights into the interactions between gut
microbes and the immune system.
In a 2012 study (Microbial
exposure during early life has persistent effects on natural killer
T cell function) published in the
journal Science, mice raised in germ-free environments showed
increased inflammation of the lungs and colon resembling asthma and
inflammatory bowel disease.
The researchers discovered that exposing
the germ-free mice to microbes normalized their immune systems and
aided in prevention of diseases.
However, this effect was observed
only in germ-free mice exposed to microbes during the first weeks of
life, but not in older germ-free mice, suggesting a strong
association between early-in-life exposure to microbes and a robust
immune system.
An imbalance of your gut microbes has also been implicated in an
inflammatory condition called increased intestinal permeability,
also known as "leaky gut."
This inflammatory state has been linked
to celiac disease and
Crohn's disease, and a recent trial in mice
suggests that leaky gut may be associated with other autoimmune
diseases, metabolic disorders, neurodegenerative diseases, and even
cancer.
Another recent study published in Cell Host & Microbe
revealed an association between a leaky gut caused by an imbalance
in gut microbes and age-associated inflammation and premature death
in mice.
Children raised in homes with dogs are less likely to develop
allergies, and researchers say the reason may be tied to their gut.
A study led by University of California, San Francisco and
University of Michigan researchers found that mice exposed to dust
from homes with dogs had a lower risk of allergies and asthma
compared to unexposed mice.
The research team traced the results to
an allergy-inhibiting gut microbe in the exposed mice called
Lactobacillus johnsonii.
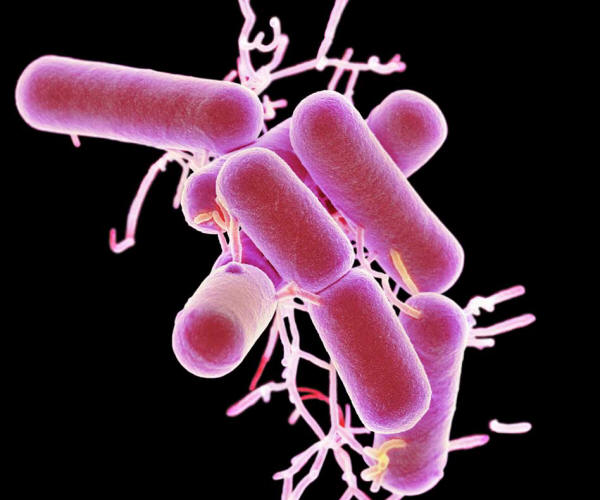
Lactobacillus bacteria,
colored scanning electron micrograph (SEM).
These bacteria are a natural
non-pathogenic component of the flora
of
the human intestines and vagina.
They produce lactic acid through
the fermentation of carbohydrates.
This helps to maintain an acidic
environment
that is hostile to pathogenic (disease-causing)
bacteria.
Getty Images/Science Photo Library
Brain and
Behavior
The
brain-gut axis, an intimate connection between the
gastrointestinal tract and the central nervous system, is one vast
and quickly emerging area of microbiome research.
This connection
relies on the
vagus nerve, a large bundle of fibers that, among its
many functions, sends bidirectional signals from the gut to the
brain.
Similar to its role in digestion, your gut microbes produce a
range of neurotransmitters, including GABA and serotonin, which can
both affect mood, appetite, and thinking, and when released from the
gut, can activate the vagus nerve.
Increasing evidence in mice have shown that certain microbes in the
gut activate the vagus nerve, and that activation plays a critical
role in mediating effects on the brain and behavior.
In one such
study, mice fed a strain of gut bacteria, Lactobacillus rhamnosus
and subjected to a number of stressful situations were found to have
less anxiety and less of the stress hormone corticosterone than mice
who were subjected to the same situations but had not been fed the
bacteria.
When the researchers snipped the vagus nerve, interrupting
the communication between the brain and the gut, the differences
between the mice disappeared.
Studies, mostly conducted in mice, have also demonstrated some
associations between gut microbes and depression and other mood
disorders, autism, and Parkinson's disease.
Snyder says that one
particularly promising area of study is the relationship between the
gut microbiome and autism.
Up to 70 percent of children and
adolescents with autism experience underlying gastrointestinal
issues, including an increased likelihood of having leaky gut, which
can produce compounds linked to altered brain function.
In
a 2013
study, researchers at Caltech reversed symptoms of leaky gut and
autism-like behavior in mice by supplying them with a gut microbe
known for its anti-inflammatory properties,
Bacteroides fragilis.
A
2016 study in pregnant and newborn mice produced similar results
using a different strain of bacteria, Lactobacillus reuteri.
Diversity
Matters, But Why?
"We don't fully know the answer," says Snyder.
"The thought is that
having a diverse microbiome means that you're making lots of
metabolites that are important to the human body."
" We do know that when some people become ill, for example, with
diabetes, their microbiome diversity tends to simplify. Yet it's
still hard to pinpoint cause and effect."
Snyder points to another possible explanation:
Short chain fatty
acids.
"One thought is that a diverse microbiome leads to lots of
short-chain fatty acids, which are thought to be very healthy for
your immune system," Snyder says.
Studies have implicated lower microbial diversity, considered a
marker of imbalance and dysfunction of the gut, in autoimmune
diseases, heart disease, obesity, as well as in age-related
inflammation and disease.
Obesity is one area of particular research interest.
In studies
comparing gut bacteria in both obese and lean animals and humans,
the gut microbes in the obese subjects tended to show less diversity
in gut bacteria.
The obese subjects also showed relatively more
pronounced levels of inflammation and insulin resistance, an
underlying cause of diabetes and a risk factor for cardiovascular
disease.
A study published earlier this year in the European Heart
Journal found a correlation between higher gut microbe diversity and
a lower risk for arterial stiffness, a contributing factor to
cardiovascular diseases in older adults.
These results and others
suggest that manipulation of gut microbes could be a useful approach
for treating or preventing obesity.
Meet Your
Microbes
Here's a short, high-level list of bacteria that scientists have
found to be present in the human microbiome, broken down by dominant
genus and a brief description of their functions in health.
In the
taxonomy of bacteria, a genus ranks a level higher and broader than
a species, for example, the all-too-familiar infection causing
bacteria
E.coli is a species of the genus Escherichia.
-
Bacteroidetes:
The most prevalent bacteria in the gut. Bacteroidetes
produce favorable metabolites, including short chain fatty
acids, which have been correlated with reducing
inflammation. Species: B. acidifaciens, B. eggerthii, B.
fragilis, B. helcogenes, B. intestinalis, and B.
thetaiotaomicron.
-
Bifidobacterium:
Bacteria found in the gut, mouth, and vagina, and also in
yogurt and some dietary supplements. It's associated with a
range of beneficial health effects, including preventing and
treating ulcerative colitis. Species: B. crudilactis, B.
denticolens, B. gallicum, B. gallinarum, B. hapali, B.
indicum, B. pullorum, and B. reuteri.
-
Lactobacillus:
Found in the mouth, gut, and vagina, and also in yogurt and
some dietary supplements. Lactobacillus has been used to
prevent and treat diarrhea and other digestive problems.
Species: L. rhamnosus, L. casei, L. fermentum, L. gasseri,
L. plantarum, L. acidophilus, and L. ultunensis.
-
Prevotella:
Found
in the gut and mouth and is associated with a plant-rich
diet. Recent research has linked Prevotella bacteria to
metabolic health. Species: P. copri, P. dentalis, P.
maculosa, P. marshii, P. oralis, P. oris, and P. salivae
-
Pseudomonas:
Found on the skin, and commonly associated with skin
infections and rashes. May also be found in the throat,
mouth, gut, urethra, and vagina. Species: P. aeruginosa, P.
maltophilia, P. aeruginosa, P. fluorescens, P. putida, P.
cepacia, and P. stutzeri.
-
Streptococcus:
Found on the skin and in the eyes, nose, throat, mouth, gut,
vagina. Associated with many illnesses, including pharyngitis, pneumonia, wound and skin infections, and
sepsis. Species: S. mitis, S. salivarius, S. mutans, S.
pneumoniae, and S. pyogenes
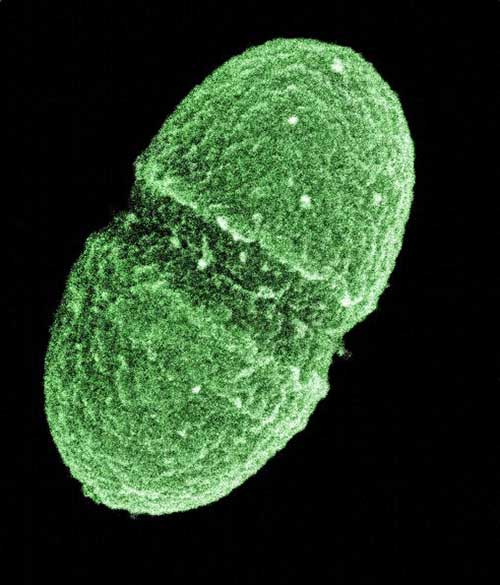
The bacterium, Enterococcus faecalis,
which lives in the human gut,
is just one type of microbe
being studied as part of
NIH's Human
Microbiome Project.
Media for Medical/UIG via Getty Images
What
Technologies are Enabling the Study of the Microbiome?
Over the last two decades, advances in sequencing technologies and
the development of
metagenomic methods have opened up new approaches
to investigating microbes and their role in health and disease.
Before sequencing, scientists relied on microscopy and culturing,
which provided a very limited picture of the microbial world.
"There's no question that whole genome sequencing has contributed
greatly to the study and cataloging of the microbiome,"
...says Snyder,
who along with his colleagues at NIH's Human Microbiome Project
created the first reference data for microbes in healthy adults
using next-generation sequencing (read more about what the data
revealed
here.)
Sequencing has allowed scientists to catalog and quantify microbial
strains and genes, and also,
"figure out whether certain microbes are
pathogenic or not," says Snyder.
"The microbiome is vast. We've found that the more you sequence, the
more you discover."
In terms of the latest in sequencing technology, Snyder says that
while researchers still rely heavily on
16S rRNA sequencing, a
popular method used for identifying and comparing microbes, more
powerful technologies have allowed for increased accuracy.
One such
technology is long-read sequencing.
"Right now we get the sequences
returned to us in fragments, and then we assemble them and make our
best guess," says Snyder.
"But with long reads, we can make much
more accurate assessments of microbes and get a better assessment of
the deviation across strains."
Another next-gen tool providing an even deeper dive into the human
microbiome is
metatranscriptomics, which allows scientists to
determine not only at what genes are present in the microbiome, but
which ones are expressed.
"And we're also looking at metaproteome to
see which proteins are made as well," says Snyder.
Move over DNA fingerprinting.
Since our microbes are specific to
each of us, our microbial fingerprint could someday potentially be
used by forensic scientists to detect our presence at the scene of a
crime.
What's Next?
Many factors from your age to what you eat to your stress levels and
the environment you live in can impact the diversity and health of
your microbiome.
In part two of this
series, we'll answer the following questions and more:
-
Can you make over your microbiome?
-
How
do antibiotics affect the microbiome?
-
Conversely, how do your
microbes affect how you respond to various drugs?
-
And how are
today's research findings translating to clinical practice, i.e.,
fecal transplants and psychobiotics?
"Until recently, the microbiome wasn't well known or understood,"
says Snyder.
"Then researchers became aware of its importance, and suddenly it
became so clear that we all have a few pounds of bacteria we're
carrying around that actually contributes a lot to your health.
That's a big deal."
The Evolution
of Microbiome Research
Studies on the diversity of the human microbiome started with Dutch
scientist
Antonie van Leeuwenhoek, when, in the 1680s, he compared
his oral and fecal microbiota.
He noted the striking differences in
microbes between these two habitats and also between samples from
individuals in states of health and disease in both of these sites.
More than three centuries and many milestones later, scientists are
still in the early days of understanding the microbiome, and
according to Blaser,
"we have barely scratched the surface."
Here's
a closer look at where it all started, from the earliest documented
studies on the microbiome and other "firsts" through where we are
today.
-
1885: The universal
model organism, Escherichia coli (formerly Bacterium coli
commune), was first described by Theodor Escherich in 1885 and
isolated from the feces of newborns in Germany.
-
1908: Russian zoologist Elie Metchnikoff theorized that health
could be enhanced and senility delayed by manipulating the
intestinal microbiome with host-friendly bacteria found in
yogurt.
-
1959: Scientists reared germ-free mice, rats, rabbits, guinea
pigs, and chicks inside sterile stainless steel and plastic
housing, known as gnotobiotic facilities, in order to study the
health of animals living in an environment completely untouched
by microbes.
-
1970: First estimate, by microbiologist Dr. Thomas D. Luckey of
the colonies of microbes living inside you: 100 billion in a
gram of human intestinal fluid or feces.
-
1995: A team of researchers led by genomics pioneer J. Craig
Venter sequenced the genome of the bacterium Haemophilus
influenzae, a common inhabitant of the human respiratory tract,
making it the first organism to have its genome completely
sequenced.
-
1996: The first human fecal sample is sequenced using 16S rRNA
sequencing.
-
2005: Researchers find bacteria in amniotic fluid of babies born
via C-section.
-
2006: The first metagenomic analysis of the human gut microbiome
is conducted.
-
2007: The NIH-sponsored Human Microbiome Project (HMP) launches
as a study to define the microbial species that affect humans
and their relationships to health.
-
2009: The first microbiome study showing an association between
the human gut microbiome and disease is conducted on obese and
lean adults.
-
2011: German researchers identify three enterotypes in the human
gut microbiome, Bacteroides, Prevotella, and Ruminococcus.
-
2012: Scientists from the HMP unveil the first "map" of microbes
inhabiting healthy humans.
-
2012: American Gut Project founded, providing an
open-to-the-public platform for citizen scientists seeking to
analyze their microbiome and compare it to the microbiomes of
others.
-
2014: The Integrative Human Microbiome Project (iHMP), the
second phase of the Human Microbiome Project begins with a goal
of studying three different microbiome-associated conditions.
-
2016: The Flemish Gut Flora Project, one of the world's largest
population-wide studies on variations in gut microbiota,
publishes its first major results, based on the analysis of more
than 1,100 human stool samples.
-
2018: The American Gut Project publishes the largest study to
date on the microbiome (American
Gut - An Open Platform for Citizen Science Microbiome
Research). The results include microbial sequence
data from 15,096 samples provided 11,336 participants across the
United States, United Kingdom, Australia, and 42 other
countries.
|