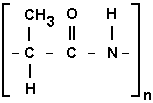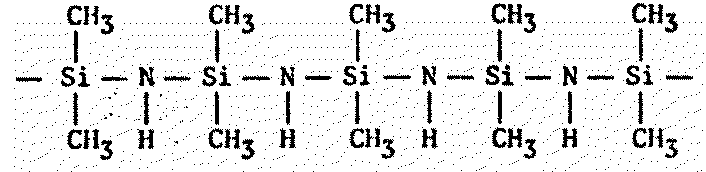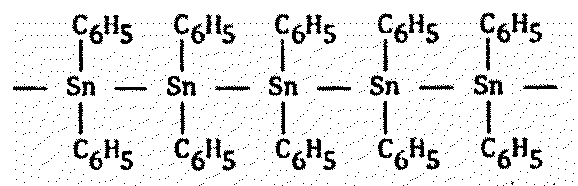8.2.1 The Limits of Carbon Aqueous
Carbon chemistry in terrestrial organisms proceeds by chemical reactions in the medium of water -- an amazing substance with a whole set of properties which make it ideal for our kind of life. Some have even contended that "water is the only possible candidate material."
In 1913, Harvard University biochemist Lawrence J. Henderson published a little book entitled The Fitness of the Environment in which he assembled for the first time the many points in favor of water as a life-fluid.879 Henderson’s analysis extends to the other molecules of life as well, and his main contribution is to show that the very chemical properties of the elements gives each of them a certain unique status and irreplaceability.
Among the many advantages of water, Henderson notes that it is an excellent solvent for countless substances, making it quite useful as a mediator of chemical activity in the liquid phase. Water, too, is an ionizing solvent, which means that an acid-base chemistry is permitted and an ever wider range of reactions can take place. (Acid-base chemistry is fundamental to Earth life but is not necessarily a requirement for all life.1074)
Hydrogen bonding between water molecules gives the liquid a high heat capacity -- the ability to store lots of heat without changing temperature very much. Organisms which use water are thus at a distinct advantage in an environment in which sudden swings between hot and cold are common. This same bonding force also holds biomolecules together so that reaction rates are enhanced64 (although it has been pointed out that H-bonding may not be absolutely essential for life2353).
Furthermore, water has a comfortably wide liquidity range -- a full 100 K under normal terrestrial conditions. However, the extent to which this temperature span may be broadened is not generally appreciated. Saturated salt water may freeze as low as 250 K; under 100-200 atm of pressure, the boiling point may be elevated to as much as 640 K.
In the proper environment, water could remain a liquid over a range of 400 degrees. It is not unreasonable to conclude that H2O may well be the solvent of choice from 250-500 K, particularly in view of its extremely high cosmic abundance.
Serious laboratory work aimed at defining and measuring the limits of carbon-based, aqueous biochemistries has just gotten under way in earnest in the 1970s. Consequently, direct evidence is only beginning to emerge from the scanty data.
In spite of this handicap, there are early signs that many alternatives are possible even within the confines of a carbon-water system.
Dr. Peter M. Molton at the University of Maryland has suggested that simple changes in the early prebiotic environment may drastically affect the chemical species which later turn up as the dominant actors on the biochemical stage of evolution.1094 His example is drawn from Miller-type experiments involving the prebiotic synthesis of amino acids, the building blocks of proteins.
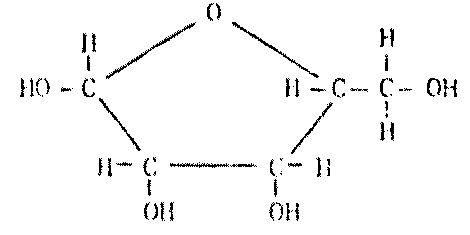
In the lab, chemists have learned that there are two common structural forms taken by amino acids. They are called alpha and beta.
The basic layout of an amino acid molecule is a chain of carbon atoms with a small -NH2 ("amino group") stuck on somewhere. In the alpha form, the amino group appears near the tail end of the molecule. In the beta form, the amino group is displaced more towards the front of the chain.
All amino acids used in terrestrial biochemistry, with one minor exception, are of the alpha variety. The beta forms are absent. Why?
Molton shows that this peculiarity may be due to nothing more complicated than the order in which water is introduced during the early stages of chemical evolution. If H2O enters into the prebiotic reactions when the first simple compounds are being synthesized, then life will evolve with proteins consisting exclusively of alpha amino acids.
This was probably the situation on the primitive Earth, eons ago.
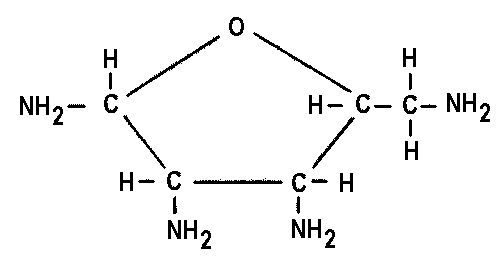
But what if the initial products of chemical evolution never come into contact with water at all in the early stages? According to Molton, when water is thus absent the beta amino acids will predominate. The proteins comprising the resulting extraterrestrial lifeforms would then be of the beta, rather than the alpha, variety.*
The next step, says Molton, is to try to synthesize plausible alternative nucleotides in the laboratory, simply by altering the prebiotic conditions under which they arise. Scientists are just beginning to see the myriad possibilities that may be open to carbon-water biochemistry on other worlds.
* Proteins made from the beta forms would probably not be edible by humans. Indeed, they might even be poisonous -- a fact of considerable importance for future interstellar astronauts and colonists.
8.2.2 Alternatives to Water
Can living processes be based on a liquid other than water (Figure 8.1)? To answer this question we must address a more fundamental problem: What are the properties of a good solvent for life?
First of all is availability. If the substance is exceedingly rare, there will not be enough of it around to sustain an ecology. Next, it should be a good solvent for both inorganic and organic compounds, and in this regard an acid-base chemistry is highly desirable. Further, the fluid ought to have a reasonably large liquidity range, so that organisms will enjoy a wide span of temperatures in which they remain biochemically operational.
A high dielectric constant is preferable -- the liquid medium should provide adequate electrical insulation from the surroundings. Also, a large specific heat would be nice, because this would give the organism thermal stability in the face of sudden or extreme temperature variations in the environment. Finally, the solvent ought to have a low viscosity -- it should not be too thick and resistant to flow (not an essential characteristic but certainly convenient).
Figure 8.1 "Ammonia! Ammonia!" (from Bracewell80)
J.B.S. Haldane, speaking at the Symposium on the Origin of Life in 1954, speculated on the possible nature of life based on a solvent of liquid ammonia.2328 The British astronomer V. Axel Firsoff picked up on this a few years later, and extended the analysis considerably.352,1217 Today, ammonia is considered one of the leading alternatives to water. Let’s see why.
Ammonia is known to exist in the atmospheres of all the gas giant planets in our solar system, and was plentiful on Earth during the first eon of its existence. Ammonia may be a reasonable thalassogen, so it should be available in sufficient quantities for use as a life-fluid on other worlds.
Chemically, liquid ammonia is an unusually close analogue of water. There is a whole system of organic and inorganic chemistry that takes place in ammono, instead of aqueous, solution.1579,1584
Ammonia has the further advantage of dissolving most organics as well as or better than water,2345 and it has the unprecedented ability to dissolve many elemental metallic substances directly into solution--such as sodium, magnesium, aluminum, and several others. Iodine, sulfur, selenium and phosphorus are also somewhat soluble with minimal reaction. Each of these elements is important to life chemistry and the pathways of prebiotic synthesis.
The objection is often heard that the liquidity range of liquid NH3 -- 44° C at 1 atm pressure -- is a trifle low for comfortable existence. But as with water, raising the planetary surface pressure broadens the liquidity range. At only 60 atm, far less than Jupiter or Venus in our solar system, ammonia boils at 98 °C instead of -33 °C. ("Ammonia life" is not necessarily "low temperature life.") So at 60 atm the liquidity range has climbed to 175 °C, which should be ample for life.
Ammonia has a dielectric constant about ¼ that of water, so it is a much poorer insulator than H2O. But ammonia’s heat of fusion is higher, so it is relatively harder to freeze at the melting point.* The specific heat of NH3 is slightly greater than that of water, and it is far less viscous (it is freer-flowing) too.
The acid-base chemistry of liquid ammonia has been studied extensively throughout this century, and it has proven to be almost as rich in detail as that of the water system (Figure 8.2). The differences between the two are more of degree than of kind. As a solvent for life, ammonia cannot be considered inferior to water.
|
INORGANIC |
HCl |
+ |
NaOH |
—> |
NaCl |
+ |
H2 |
Aqueous-life chemistry |
| HOH |
+ |
NaNH2 |
—> |
NaOH |
+ |
NH3 |
Ammonia-life chemistry |
|
| ORGANIC |
CH3COOH |
+ |
NaOH |
—> |
CH3COONa |
+ |
H2 |
Aqueous-life chemistry |
| CH3CONH2 |
+ |
NaNH2 |
—> |
CH3CONHNa |
+ |
NH3 |
Ammonia-life chemistry |
|
| Acid |
+ |
base |
—> |
Salt |
+ |
Solvent |
||
Compelling analogues to the macromolecules of Earthly life may be designed in the ammonia system. But Firsoff has urged restraint: An ammonia-based biochemistry might well develop along wholly different lines. There are probably as many different possibilities in carbon-ammonia as in carbon-water systems.1172
The vital solvent of a living organism should be capable of dissociating into anions (negative ions) and cations (positive ions), which permits acid-base reactions to occur (Table 8.3). In the NH3 solvent system, acids and bases are different than in the water system-acidity and basicity, of course, are defined relative to the medium in which they are dissolved.
Table 8.3 Dissociation of the Vital Solvent1217
| Solvent |
Anions |
Cations |
|||
|---|---|---|---|---|---|
| H2 |
OH - |
O= |
H + |
H3O+ |
|
| NH3 |
NH2- |
NH = |
N º |
H + |
NH4+ |
In the ammonia system, water, which rests with liquid NH3 to yield NH4+ ion, would seem as a strong acid, quite hostile to life. Ammono-life astronomers, eyeing our planet from their chilly observatories, would doubtless view the beautiful, rolling blue oceans of Earth as little more than "vats of hot acid."
After all, water and ammonia are not chemically identical. They are simply analogous. There will necessarily be many differences in the biochemical particulars. Molton has suggested, for example, that ammonia-based lifeforms may use cesium and rubidium chlorides to regulate the electrical potential of cell membranes. These salts are more soluble in liquid NH3 than the potassium or sodium salts used by Earth life.1132
Dr. Molton concludes: Life based on ammonia instead of water is certainly possible (Figure 8.2), theoretically, at the superficial level. If we delve further into the complex biochemistry of the cell, we could find some insuperable barrier to ammonia-based life -- but it is hard to conceive of any obstacle so insuperable that it would rule it out altogether.
|
Biochemical Type |
Terrestrial Water-Life Form |
Possible Ammonia-Life Analogue |
|---|---|---|
|
Typical Alcohol |
H H |
H H |
|
Typical Fatty Acid |
H O |
H O |
|
Typical Amino Acid |
H H O |
H H O |
|
Typical Protein Polymer |
|
|
|
Typical Carbohydrate |
|
|
There are many other life-solvents (Table 8.4) which have been studied to varying degrees, though none so extensively as ammonia. Hydrogen fluoride (HF), for instance, has often been proposed. HF is an excellent solvent in theory both for inorganics and organics vital to carbon-based life.
Hydrogen fluoride has a larger liquidity range than water and has hydrogen bonding as well as an acid-base chemistry (in which nitric and sulfuric acids act as bases!).1583 It also has a large dielectric constant and a sizable specific heat. The major difficulty with HF is its extreme cosmic scarcity. However, this need not be a fatal objection in view of the widespread use of the equally rare element phosphorus in terrestrial biochemistry.
Liquid hydrogen cyanide (HCN) is another possibility. Unlike HF, hydrogen cyanide has a reasonably high cosmic abundance -- although it still may be too low to be of xenobiochemical significance. HCN is a good inorganic and organic solvent, has an adequate liquidity range, has hydrogen bonding, a large dielectric constant and specific heat, and a viscosity five times lower than that of water. Its chemistry, however, may be complicated by its tendency to polymerize.
Hydrogen sulfide (H2S) is the sulfur analogue of water, in which S atoms replace those of oxygen. (The two elements are of the same family in the Periodic Table (Table 8.5), and have similar chemical properties.) We might expect that H2S would have similar solvating abilities to water, but such is not the case. Hydrogen sulfide has only weak hydrogen bonding, a low dielectric constant, and is a very poor inorganic solvent.1578 Its narrow liquidity range (25 °C) means that it should be suitable, if at all, only for planets with heavy atmospheres and small daily temperature variations.
| |
||||||||
|---|---|---|---|---|---|---|---|---|
|
H |
|
|
|
|
|
|
He |
First |
|
Li |
Be |
B |
C |
N |
O |
F |
Ne |
Second |
|
Na |
Mg |
Al |
Si |
P |
S |
Cl |
Ar |
Third |
|
K |
Ca |
Ga |
Ge |
Ar |
Se |
Br |
Kr |
Fourth |
|
Rb |
Sr |
In |
Sn |
Sb |
Te |
I |
Xe |
Fifth |
|
Os |
Ba |
Ti |
Pb |
Bi |
Po |
At |
Rn |
Sixth |
|
Sodium Group I |
Calcium Group II |
Boron Group III |
Carbon Group IV |
Pnictide Group V |
Chalcogen Group VI |
Halogen Group VII |
Noble Gas Group VIII |
|
Sulfur dioxide, another possible thalassogen, is an ionizing substance which is a good organic and a fair inorganic solvent. It has an adequate liquidity range, but a very low dielectric constant.
Carbon disulfide, a wide liquidity range fluid, solvates sulfur and a number of organic compounds. But it is relatively unstable with heat and is expected to be rare on most planetary surfaces.
Little is known about chemistry in liquid chlorine (Cl2). While it has a good liquidity range, it is five times more viscous than water. One peculiar halogen hybrid, fluorine oxide (F2O), is a direct analogue of water. This intensely yellow fluid is a good ionizing solvent, unstable at high temperatures but ideal for biochemistry below 100 K. At such temperatures, F2O might serve as solvent for the coordination chemistry of the noble gases.1172
There are many, many other less likely solvents that have been discussed in the literature.**
* The point is sometimes made that water has the virtually unique property of expanding upon freezing, which means that ice will float atop a cooling mass of water and protect the lifeforms beneath. However, water freezing within the cells of living tissue exposes the organism to a new hazard -- mechanical damage by expansion. Since ammonia shrinks when it freezes, the very property responsible for massive oceanic freeze-ups should also allow ammono lifeforms to be much more successful hibernators in a frozen clime.
** Dr. Allen M. Schoffstall at the University of Colorado at Colorado Springs has performed some preliminary experiments with possible prebiotic syntheses in exotic solvents, such as formic acid, acetic acid, liquid formamide and other nonaqueous solvents. His experiments have demonstrated the feasibility of prebiotically converting nucleosides to nucleotides or nucleoside diphosphates in anhydrous liquid formamide -- an alternative solvent to water.2384 Similar research is just now getting started at several other laboratories.4086
8.2.3 Alternatives to Carbon
Why do lifeforms prefer carbon?
Few elements can compete with its ability to combine with many different kinds of other atoms. As for its ability to form long, polymeric chains, carbon knows no equal. There are many who believe that the element is "uniquely qualified" for the job of life. They may well be correct.
The idea of living systems founded on a radically different chemical basis from ours has been around for a long time. It was already old hat in 1908 when Dr. J.E. Reynolds, a British biochemist, delivered a paper on the subject at a meeting of the Royal Institution in London. The reviewer for Chemical Abstracts wearily reported:
... It contains no new matter. The author advances a speculative theory as to the probability of a "high temperature protoplasm" containing silicon in place of carbon and phosphorus in place of nitrogen, and points out that silicon found in certain animal and plant cells may actually be a constituent of the protoplasm of such cells.1608
Among xenologists, the possibility of silicon (Si) -based extraterrestrial lifeforms was raised by the British astronomer Sir Harold Spencer Jones as early as 1940.44 In more recent times, silicon-based structures have become perhaps the best-known and most commonly advanced proposal as an exotic biochemistry for aliens (Figure 8.3).
Figure 8.3 Depiction of a silicon-based lifeform in science fiction
The Horta, a silicon-based lifeform depicted in an episode of Star Trek, crouches in fear of the approaching humans. The small mineral nodules littering the subterranean lair are the creature’s eggs.
This is because Si lies directly below C in the Carbon Family of the Periodic Table of the Elements (Table 8.5). Members of the same family are expected, more or less, to have similar chemical properties and to form analogous compounds.
There have been numerous objections to silicon life from all quarters of the scientific community.
A common protest, for example, is based on the relative cosmic scarcity of Si as compared to C. From Table 8.6, we note that carbon is roughly an order of magnitude more abundant than silicon in the universe.
| |
||||||||
|
Atomic Number |
Element |
Symbol |
Abundances of Atoms |
Atomic |
Element |
Symbol |
Abundances of Atoms |
|
|---|---|---|---|---|---|---|---|---|
| 1 |
Hydrogen |
H |
31800000. |
44 |
Ruthenium |
Ru |
0.0019 |
|
| 2 |
Helium |
He |
2210000. |
45 |
Rhodium |
Rh |
0.0004 |
|
| 3 |
Lithium |
Li |
0.0495 |
46 |
Palladium |
Pd |
0.0013 |
|
| 4 |
Beryllium |
Be |
0.00081 |
47 |
Silver |
Ag |
0.00045 |
|
| 5 |
Boron |
B |
0.350 |
48 |
Cadmium |
Cd |
0.00148 |
|
| 6 |
Carbon |
C |
11800. |
49 |
Indium |
In |
0.000189 |
|
| 7 |
Nitrogen |
N |
3740. |
50 |
Tin |
Sn |
0.0036 |
|
| 8 |
Oxygen |
0 |
21500. |
51 |
Antimony |
Sb |
0.000316 |
|
| 9 |
Fluorine |
F |
2.45 |
52 |
Tellurium |
Te |
0.00642 |
|
| 10 |
Neon |
Ne |
3440. |
53 |
Iodine |
I |
0.00109 |
|
| 11 |
Sodium |
Na |
60. |
54 |
Xenon |
Xe |
0.00538 |
|
| 12 |
Magnesium |
Mg |
1061. |
55 |
Cesium |
Cs |
0.000387 |
|
| 13 |
Aluminum |
Al |
85. |
56 |
Barium |
Ba |
0.0048 |
|
| 14 |
Silicon |
Si |
1000. |
57 |
Lanthanum |
La |
0000445 |
|
| 15 |
Phosphorus |
P |
9.6 |
58 |
Cerium |
Ce |
0.00118 |
|
| 16 |
Sulfur |
S |
500. |
59 |
Praseodymium |
Pr |
0.000149 |
|
| 17 |
Chlorine |
Cl |
5.7 |
60 |
Neodymium |
Nd |
0.00078 |
|
| 18 |
Argon |
Ar |
117.2 |
62 |
Samarium |
Sm |
0.000226 |
|
| 19 |
Potassium |
K |
4.2 |
63 |
Europium |
Eu |
0.000085 |
|
| 20 |
Calcium |
Ca |
72.1 |
64 |
Gadolinium |
Gd |
0.000297 |
|
| 21 |
Scandium |
Sc |
0.035 |
65 |
Terbium |
Tb |
0.000055 |
|
| 22 |
Titanium |
Ti |
2.775 |
66 |
Dysprosium |
Dy |
0.00036 |
|
| 23 |
Vanadium |
V |
0.262 |
67 |
Holmium |
Ho |
0.000079 |
|
| 24 |
Chromium |
Cr |
12.7 |
68 |
Erbium |
Er |
0.000225 |
|
| 25 |
Manganese |
Mn |
9.3 |
69 |
Thulium |
Tm |
0.000034 |
|
| 26 |
Iron |
Fe |
830. |
70 |
Ytterbium |
Yb |
0.000216 |
|
| 27 |
Cobalt |
Co |
2.21 |
71 |
Lutetium |
Lu |
0.000036 |
|
| 28 |
Nickel |
Ni |
48. |
72 |
Hafnium |
Hf |
0.00021 |
|
| 29 |
Copper |
Cu |
0.54 |
73 |
Tantalus |
Ta |
0.000021 |
|
| 30 |
Zinc |
Zn |
1.244 |
74 |
Tungsten |
W |
0.00006 |
|
| 31 |
Gallium |
Ga |
0.048 |
75 |
Rhenium |
Re |
0.000053 |
|
| 32 |
Germanium |
Ge |
0.115 |
76 |
Osmium |
Os |
0.00075 |
|
| 33 |
Arsenic |
As |
0.0066 |
77 |
Iridium |
Ir |
0.000717 |
|
| 34 |
Selenium |
Se |
0.0672 |
78 |
Platinum |
Pt |
0.0014 |
|
| 35 |
Bromine |
Br |
0.0135 |
79 |
Gold |
Au |
0.000202 |
|
| 36 |
Krypton |
Kr |
0.0468 |
80 |
Mercury |
Hg |
0.0004 |
|
| 37 |
Rubidium |
Rb |
0.00588 |
81 |
Thallium |
11 |
0.000192 |
|
| 38 |
Strontium |
Sr |
0.0269 |
82 |
Lead |
Pb |
0.004 |
|
| 39 |
Yttrium |
Y |
0.0048 |
83 |
Bismuth |
Bi |
0.000143 |
|
| 40 |
Zirconium |
Zr |
0.028 |
90 |
Thorium |
Th |
0.000058 |
|
| 41 |
Niobium |
Nb |
0.0014 |
92 |
Uranium |
U |
0.0000262 |
|
| 42 |
Molybdenum |
Mo |
0.004 |
|||||
But the real business of biochemical evolution takes place on planetary surfaces. The Earth, Moon, and Mars are remarkably similar in their silicon content -- roughly 25-30% of the total topsoil. But on this planet, Si atoms outnumber those of C by more than two orders of magnitude (Table 8.7). Organics are present in lunar soil only to the extent of a few parts per million, and on Mars there is no trace of carbon in the crust even at the parts-per-billion level.
| |
||||
|---|---|---|---|---|
| (weight %) |
||||
|
Element |
Universe |
Earth’s Crust |
Seawater |
Human Body |
|
Hydrogen |
91. % |
0.22 % |
66. % |
63. % |
|
Helium |
9.1 % |
0.0000003 % |
-- |
-- |
|
Oxygen |
0.063 % |
47. % |
33. % |
25.5 % |
|
Carbon |
0.035 % |
0.19 % |
0.0014 % |
9.4 % |
|
Nitrogen |
0.011 % |
0.015 % |
0.000745 % |
1.4 % |
|
Neon |
0.010 % |
-- |
-- |
-- |
|
Magnesium |
0.0031 % |
2.2 % |
0.033 % |
0.013 % |
|
Silicon |
0.0029 % |
28. % |
0.00011 % |
-- |
|
Iron |
0.0024 % |
4.5 % |
0.0000005 % |
0.0038 % |
|
Sulfur |
0.0015 % |
0.026 % |
0.017 % |
0.049 % |
|
Aluminum |
0.00025 % |
7.9 % |
0.000014 % |
-- |
|
Calcium |
0.00021 % |
3.5 % |
0.006 % |
0.3 % |
|
Sodium |
0.00018 % |
2.5 % |
0.28 % |
0.041 % |
|
Phosphorus |
0.000028 % |
0.026 % |
0.0000016 % |
0.21 % |
|
Chlorine |
0.000017 % |
0.032 % |
0.33 % |
0.026 % |
|
Potassium |
0.000012 % |
2.5 % |
0.006 % |
0.057 % |
|
Titanium |
0.000008 % |
0.46 % |
0.00000014 % |
-- |
A few have suggested that since carbon-based Earth life exhales carbon dioxide, a gas, silicon-based lifeforms must surely "breathe out silicon dioxide, SiO2, which is quartz: a painful process . . ."49 It is difficult to find any merit to this biochauvinistic objection. Silicon organisms probably are able to survive only in a reducing, oxygen-free environment -- so SiO2 should not be produced at all. Even if it is, it’s not clear why an extraterrestrial lithomorph should find the excretion of sand at all painful.
A seemingly more valid challenge is the contention that any available prebiotic silicon atoms will be irreversibly locked into large, heavy SiO2 polymers, making it impossible for them to participate in any life chemistry. But silicon dioxide is far from absolutely stable. In fact, it is the original material in the synthesis of many silicon-organic molecules under the action of various chemical reagents.26
Another common complaint is that the number of carbon compounds catalogued -- perhaps two million or so -- greatly exceeds the total number of silicon-based substances known to chemists today -- about 20,000, two orders of magnitude less.
But the only reason a class of compounds is found may be because someone went looking for them. As few as twenty-seven organosilicon molecules were known at the turn of the century, and real interest in silicon chemistry began to accelerate just a few decades ago. Furthermore, the pitiful number of scientists currently engaged in silicon research is dwarfed by the armada of pharmaceutical houses and petrochemists flying the flag of carbon.
As Carl Sagan notes with some amusement: "Much more attention has been paid to carbon organic chemistry than to silicon organic chemistry, largely because most biochemists we know are of the carbon, rather than the silicon, variety."15
The inability of lone Si atoms to readily hook together to form very long chain polymers is often cited as the fatal flaw in all silicon biochemistry schemes. But exactly how crucial is this ability to concatenate?
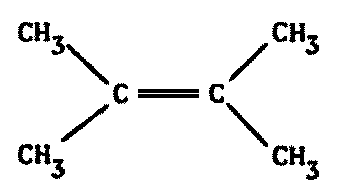
In Earthly proteins, carbohydrates, and nucleic acids -- the three most important and common polymer types -- the C-C linkages rarely include more than a few consecutive atoms. Organic side chains may contain up to eight, and fats and various vitamin complexes use even more successive carbons, but the basic molecular backbone of life is served by only a few. For instance, most proteins consist of a repeating -C-C-N- unit, a mere two carbons in a row.
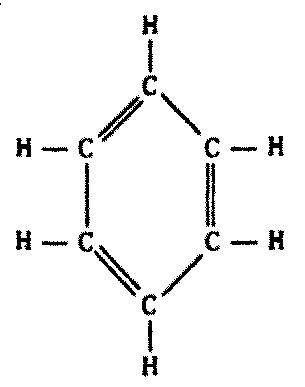
Biochemistries need stable polymers, not long chains of similar backbone atoms (Figure 8.4).
Silicon, in combination with nitrogen and oxygen, forms a variety of ring-shaped and chain-polymer macromolecules stable in high ultraviolet radiation fluxes (such as might be found near a class F star or on the surface of an unshielded planet like Mars) and at low temperatures as well.1597 Silane (SiH4), the silicon analogue of methane with a repulsive odor, remains a liquid between 88.l K and 161.4 K. It might serve as a solvent for a cold silicon biochemistry under anhydrous reducing conditions. The Si halides might also work, though at somewhat higher temperatures.
Unfortunately, Si-Si bonds tend to break up in the presence of ammonia, oxygen, or water, all of which are more likely to appear on a colder world. This difficulty disappears in a hot environment in which the role of oxygen has been usurped by its chemical cousin, sulfur. The problem then becomes one of preventing the low-energy Si-Si bonds from tearing themselves to pieces in the blistering heat.1172
At present, the biggest obstacle is in devising plausible pathways of prebiotic evolution (Figure 8.5). Carbon seems more competitive under most conditions we can readily imagine.** Yet as Dr. Molton says, "this may be due to our own ignorance of silicon chemistry as much as to any inherent theoretical difficulty."1132
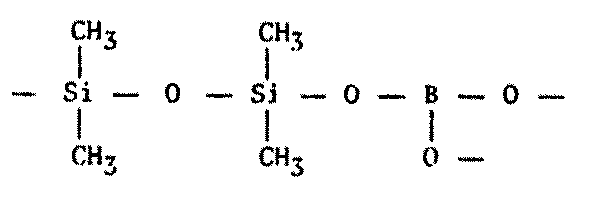
In the last few decades a broad, new class of silicon polymers has been discovered which might serve as a basis for life. These substances, known as siloxanes to the chemist and as "silicones" in popular parlance, are extremely stable in the presence of oxygen and water. In fact, many silicones are formed by the action of water on the Si-Si bond.
This novel class of compounds is now under intensive investigation, as they have been found to exhibit a wide range of fascinating properties. There are rubbery silicones, analogous to soft living tissue, which remain flexible and "elastomeric" across a span of temperature that few organic polymers can match. There are hard silicone resins with impact and tensile strengths comparable to those of bone, and which retain their stoutness in hot environments.1607,1610
Silicone liquids are useful as hydraulic fluids, and some of them have very handy peculiarities. For example, polydimethylsiloxane is an oil with variable mechanical properties strikingly similar to those of mammalian synovial fluid (a kind of bone joint lubricant).230
Some silicone rubbers are selectively permeable to specific gases. One rubber which passes oxygen has been tested in artificial gill devices designed to extract the dissolved gas from seawater for the benefit of human divers.2348 These compounds are generally less active chemically, stronger, more heat-resistant and more durable than their carbon counterparts.
The molecular architecture of the silicones is relatively simple. Silicones have a backbone, not of Si atoms alone but rather of alternating silicon and oxygen atoms. The side chains can be organic, and are as complicated as any in terrestrial organic chemistry. Silicones appear to possess an information-carrying capacity and a complexity of structure as required for a successful biochemistry.
There remain two problems with such silicon-oxygen lifeforms, which must be dealt with before the plausibility of their existence can be acknowledged.
First, many silicones tend to disassemble into ring molecules at temperatures of roughly 300-350 °C. (Similar behavior is observed in most complex carbon compounds, but at somewhat lower temperatures.) It would be difficult for silicones to remain stable in much hotter climes, and it is unclear whether this slight thermal advantage is enough to enable Si to out-compete C in a high temperature regime.
There do exist a few silicon polymers that can really get out of carbon’s league. Certain Si-C combinations are good to at least 500 °C, and various aluminum-silicon structures can reach 600 °C without destruction.
The second problem that must be faced is a familiar one: How do we arrange for a plausible prebiotic evolutionary sequence? Natural planetary conditions, by and large, are not conducive to the prebiotic synthesis of silicones.
Worse, recall that most of the complexity of the silicones is derived from the carbon side chains they possess. In spite of their greater thermal stability, these Si polymers may find themselves in an indirect competition with carbon-based macromolecules.
On any world in which the carbon chemistry had evolved sufficiently far to allow C side chains (as on the Si backbone) of the requisite complexity, it is far more likely that these carbon chains would form polymers among themselves rather than splicing onto an "alien" silicone backbone molecule.
Of course, silicon is not the only game in town. Other members of the Carbon Family might stand in for C, although this is much less likely.
Germanium has been suggested as an analogue to carbon in some biochemical systems. N.W. Pirie has cited some rather dubious evidence for germanium-based protobionts in Earth’s past: The excessive concentration of Ge in the Hartley coal seam in Northumberland, England.2347
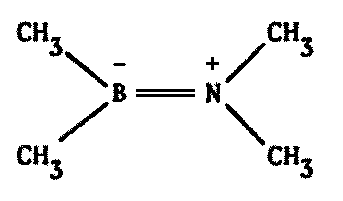
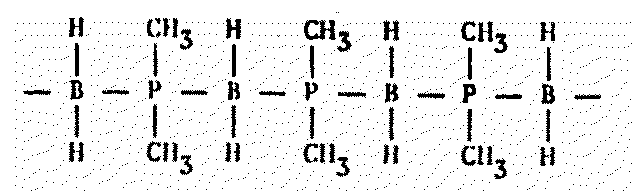
But we are not restricted to the Carbon Family in our quest for analogues to C. One alternative not widely known outside specialist circles involves a tricky arrangement with the element boron (B).1172,2089,2446
Looking at the Periodic Table, we see that boron lies just to the left, and nitrogen just to the right, of carbon. One might well suspect that a kind of averaging effect could take place if the two elements were combined, resulting in some sort of "pseudocarbon" system.
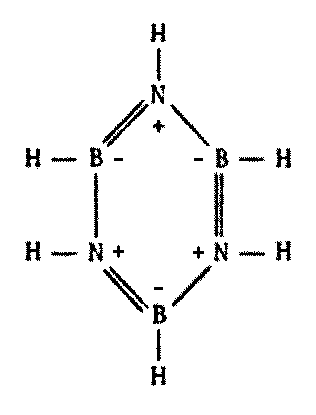
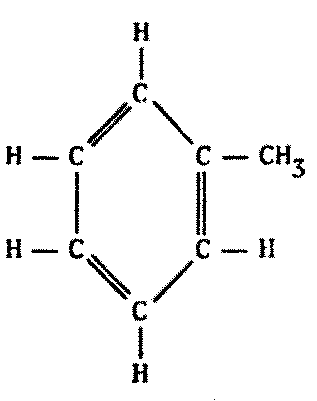
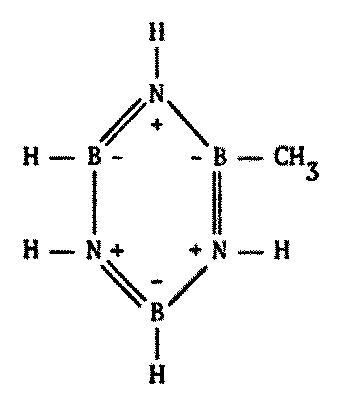
Indeed, this does occur. There are compounds made of alternating boron and nitrogen atoms which closely parallel their organic counterparts in many ways. They have the same types of bonds, similar molecular weights, similar physical and chemical properties, and so forth. A few possibilities are illustrated on the following page by comparing a series of common carbon compounds with their boron-nitrogen analogues (Figure 8.6).
While some B-N polymers are known to be stable to high temperatures, many such substances turn out to be less stable with heat. Borazine, the boron-nitrogen analogue to benzene, is more susceptible to chemical attack because of its greater reactivity. The presence of water tends to degrade most B-N polymeric compounds.
Part of these difficulties can be eliminated by switching to other combinations which also give a "pseudocarbon" effect. There are the boron-phosphorus (borophane) and the boron-arsenic (boroarsane) systems, which are known to be extraordinarily stable and inert to thermal decomposition. These substances might serve on high temperature worlds if the abundance problem could be licked.
A completely different kind of exotic biochemistry is the possibility of halogen life. Members of the Halogen Family, of which fluorine and chlorine are the most abundant, could conceivably replace hydrogen atoms in whole or in part. This would apply to biological macromolecules constructed on the basis of carbon, silicon, or any other viable backbone system.
An oxygen-poor star might give rise to planets with abnormally high concentrations of free halogen. This is not as unreasonable as it might sound at first. The element phosphorus, a common atom in Earthly biochemistry, has a cosmic abundance approximately equal to that of fluorine and chlorine. Thus, the availability and use of halogens by alien lifeforms cannot be categorically ruled out.
There might exist water oceans and an atmosphere rich in chlorine or fluorine. Peter Molton has proposed a respiration-photosynthesis cycle for such a world, involving carbon tetrachloride as the halogen analogue of methane.1132
Going still further out on a limb, Isaac Asimov has set forth the possibility of fluorocarbon (Teflon) or chlorocarbon polymers floating in seas of molten sulfur. "No one," the Doctor gently chides, "has yet dealt with the problem of fluoroproteins or has even thought of dealing with it."2344 No one, that is, except science fiction writers.1359
Actually, polymers of any kind should be of interest to xenobiologists (Figure 8.7). Since the basis of all life appears to be the polymeric organization of small molecules into larger ones, polymer chemistry seems a reasonable avenue to explore for alternative biochemistries.
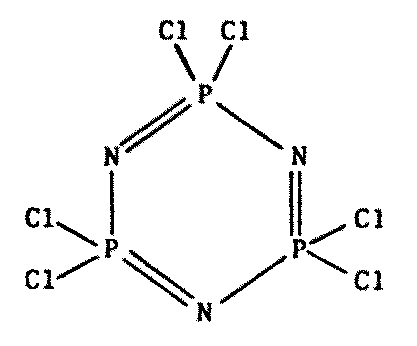
Figure 8.7 Other Polymers of Possible Xenobiochemical Interest
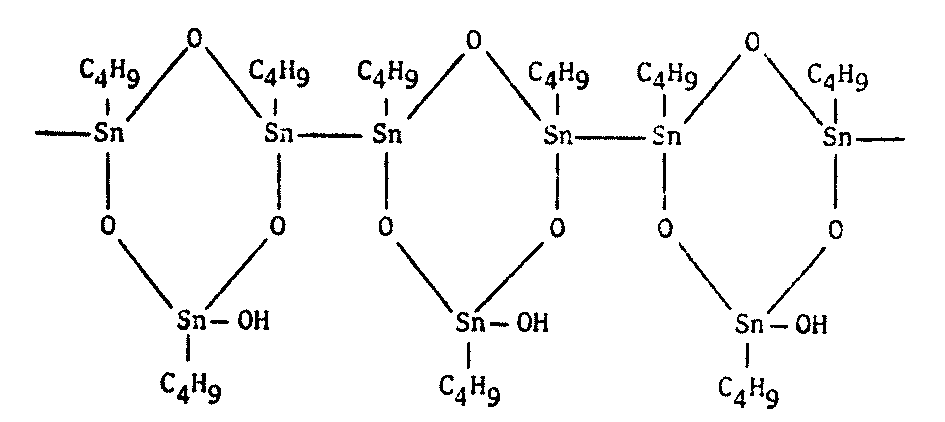
| |
|
|
| Cyclosilazanes1599 |
Cyclosilthians1600 |
|
| |
|
|
| Polysilazanes1599 |
Polymeric diphenyltin1603 |
|
|
|
||
| "Random" silicon-aluminum copolymer1603 |
||
|
Cl Cl
Cl |
|
|
| Polymeric phosphonitrilic chloride1603 |
Polyphosphazine chloride trimer2348 |
|
|
CH3HN CH3HN
CH3HN |
SUBSTITUTED |
CF3CH2O CH3CH2O
CF3CH2O |
| (a water-soluble polymer)2348 |
(film-forming flexible crystalline thermoplastic)2348 |
|
| |
BORON |
|
| Dimethyl polyborophanes1574 |
Polymeric silyl orthoborates1573 |
|
In view of various deficiencies in normal carbonaceous organic chains, many other classes have been examined in recent times.2348 According to H. R. Allcock, a chemist at Pennsylvania State University, "a new revolution based on organic polymers is about to begin."
Silicon-nitrogen rubbers and oils have been known for many years. These compounds, called silazanes, are unstable in the presence of water or in an oxygen atmosphere.1598 Inorganic polymers with alternating silicon and boron atoms have turned up recently, and a boron-oxygen-silicon linkage is used in the well-known "silly putty." Various carbon-boron ("carborane") polymers which are quite stable have been discussed in the literature,1575 along with short-chain nitrogen, sulfur, and silicon-sulfur arrangements.
Phosphorus, nitrogen, and chlorine combine to form a kind of rubber in a water-free environment. These "polyphosphazines," as the chemists love to call them, are normally highly unstable in the presence of H2. However, it has recently been learned that short segments can be polymerized and made water-stable.
Soon after this discovery, the elated researchers wrote: "... it now seems likely that almost any set of required properties can be designed into the polymer by a judicious choice of side groups." The proposal that polyphosphazine polymers be used in biomedical applications to transport fixed metal ions2351 suggests a wide range of xenobiochemical applications, perhaps analogous to the metal-containing complexes in chlorophyll and hemoglobin.
* While more than thirty carbonaceous molecules have been detected in the interstellar void by radioastronomers, only two silicon compounds -- the monoxide (SiO) and the sulfide (SiS) -- had been found as of 1976.1002 This may, however, reflect more the zeal and interests of the searchers than the true ubiquity of molecular species containing silicon.
** It should be noted that partial substitution of Si for C occurs even in terrestrial skeletal components (e.g., diatoms, some grasses, etc.) and in protoplasm.1551,1649 Dr. Alan G. MacDiarmid, Professor of Chemistry at the University of Pennsylvania, has succeeded in forcing bacteria to take up silicon analogues of various carbon compounds in their nutrients. He has conducted similar experiments using analogues based on germanium (Ge),1172 the element directly below silicon in the Periodic Table and whose compounds have long been known to possess certain medical properties.1576