|
from Nature Website
through tunneling nanotubes.
Networks of nanotubes may allow cells to share everything from infections and cancer
to dementia-linked proteins.
But when she carried out
a set of experiments on the organ five years ago, it ended up
leaving her flummoxed.
But instead of showing up in the
engineered cells, some proteins seemed to have teleported to a
different group of cells entirely.
They were convinced it was real - but they couldn't understand how it worked.
So they shelved the project until one day, more than a year later, Inaba presented Yamashita with some images of 'tiny tubes' reaching out from one cell to another - delicate structures that might have been responsible for the trafficking.
Yamashita was skeptical, but decided to dig out images from her own postdoc project 12 years earlier.
Sure enough, slender spikes jutted out towards the targeted cells.
The group published its work in 2015, arguing that the tubes help testis cells to communicate precisely, sending a message to some of their neighbors and not others. 1
Longer tubes, reported in mammalian cells, seem to transport not just molecular signals but much larger cargo, such as viral particles, prions or even mitochondria, the cell's energy-generating structures.
These observations suggest an unanticipated level of connectivity between cells, says Amin Rustom, a neurobiologist at the University of Heidelberg in Germany, who first spotted such tubes as a graduate student almost 20 years ago.
If correct, he says,
But Richard Cheney, a cell biologist at the University of North Carolina in Chapel Hill, is not ready to start revising the textbooks.
Cheney has followed the field and at one point collaborated with Rustom's PhD adviser. There's no question that long, thin protrusions are popping up all over the place, he says.
The question is,
The problem with betting either way is that these tiny tubes are tough to study.
Arguing that they exist at all is hard enough, let alone making the case that they actually have a function.
Yamashita used the tried-and-tested genetic-engineering methods and well-characterized genes available in the fruit fly to argue that her tubes were sending signals by direct contact.
But researchers looking for tubes in mammalian cells don't have those resources. More than one researcher has been accused of mistaking a scratch on a cell plate for a cell-produced nanotube.
Evidence derived from real mammalian tissue is even sparser.
takes a closer look at the tiny tubes spotted between cells.
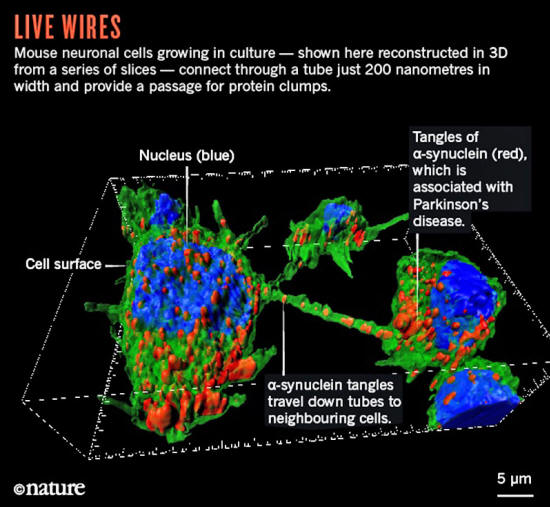
One of the believers is George Okafo, a director of emerging platforms at the drug company GlaxoSmithKline (GSK) in Stevenage, UK.
He thinks that cell-to-cell protrusions could explain why diseases such as,
...are so difficult to treat (see below image 'Live Wires').
Pasteur Institute
Last September, Okafo organized an invitation-only conference to bring together GSK staff and around 40 researchers in the field. (He is now collaborating with some of them.)
In March this year, the US National Institutes of Health asked for grant applications from groups studying how organelles communicate in stressed or cancerous cells, a move that excites tube enthusiasts.
And in December, the
American Society for Cell Biology will host a session devoted to the
topic at its annual meeting.
The first important hint that they might be involved in something more complex came in 1999, from cell biologist Thomas Kornberg at the University of California, San Francisco.
He was watching fly larvae develop wings, and saw a sea of filaments projecting from the wing buds towards the signaling centre that is essential for their growth. 2
He coined the term cytoneme - or cell thread - to describe these filaments.
He suggested that some
cellular chatter that was thought to happen by diffusion could, in
fact, be orchestrated by cytonemes. The idea was surprising and was
slow to catch on, but it is now making its way into textbooks.
Rustom spotted thin, straight tubes connecting cultured rat cells after he forgot a washing step in an experiment.
He and his adviser at the University of Heidelberg, Hans-Hermann Gerdes, engineered cells to make fluorescent proteins and watched the molecules flow from one cell to another.
Their accidental sighting
grew into a Science paper 3 that described the structures
as "nanotubular highways". (Some skeptics think that Gerdes chose
the term nanotube to ride on the coat-tails of carbon
nanotubes, a
hot topic in materials science.)
Davis attributes their discovery to his team's willingness to think through the implications of their sighting.
His team went on to
describe different sorts of nanotube, some holding vesicles and
mitochondria inside, and others with bacteria 'surfing' the casing.
5
Further types of tube have been spotted as well.
In 2010, Gerdes and his team reported that some tubes end in gap junctions:
Yamashita speculates that such connections may be more than conceptually related to neuronal synapses.
Most researchers who study these cellular pipelines care less about their evolutionary origin than about their role in human health and disease.
The strongest evidence for a role in disease came in 2015, also from a team at the University of Heidelberg, led by cancer researcher Frank Winkler.
Like others, his team had not set out to study cell protrusions; they wanted to test a system for watching human gliomas grow.
Cells derived from the
tumors were injected into the brains of mice with windows in their
skulls - hardened glass kept in place with dental cement - through
which the researchers could watch the cells.
A closer look showed many tubes connecting cells through gap junctions. Interconnected cells managed to survive doses of radiation that killed isolated cells, apparently because gap junctions helped to spread the load of toxic ions to neighbors. 7
When radiation did kill linked tumor cells, nuclei from those cells sometimes travelled down a tube, with the tube then expanding into the cleared space to form a vigorous new cancer cell.
These 'tumor microtubes' were also found in biopsies from patients, and denser, longer tubes correlated with more resistant forms of cancer and a poorer prognosis.
Winkler speculates that a drug that could keep these tubes from sprouting or extending might create a new class of cancer treatment; indeed, he thinks that existing cancer drugs such as paclitaxel may work by disrupting tumor microtubes.
Winkler's team has filed
a patent application for a compound that interferes with microtubes
as a treatment for
glioma.
But it's not clear whether Winkler's results apply to other scenarios.
Various sorts of brain cell are known to send out cell protrusions as they grow and proliferate. The tubes that Winkler's team reported are much larger than the 'tunneling nanotubes' that were originally described by Gerdes, and, unlike most tunneling nanotubes reported so far, contain microtubules - filaments that move components around in cells.
However, Winkler thinks that his work provides evidence for a broad role for tunneling-nanotube-like structures. He thinks they may not be able to reach full size in culture, and the tubes he does see vary considerably in length and thickness.
Winkler recalls discussing his work with Gerdes before Gerdes' death in 2013.
In other fields, too, the tubes are gaining traction.
Eliseo Eugenin, who studies HIV at Rutgers New Jersey Medical School in Newark, suggests that HIV-infected cells send out multiple nanotubes filled with virus to reach uninfected cells.
Circulation and one-on-one cellular contact would be too inefficient to cause the rapid amplification of the virus seen in newly infected patients.
He thinks that other researchers are skeptical of nanotubes because they are unable to reconcile themselves to the idea that cells are constantly exchanging materials, including genetic information.
Emil Lou, a cancer
researcher at the University of Minnesota in Minneapolis, says his
grant proposal to hunt for and characterize nanotubes in human
cancers was pooh-poohed because a reviewer was not convinced that
the structures existed.
Michael Dustin, an immunologist at the University of Oxford, UK, says that he has seen cells in dishes form structures that would never occur in the dense tissue of an organism.
For example, white blood cells primed to produce antibodies produce a "beautifully symmetric" bull's-eye pattern in a dish, very different from the chaos and asymmetry they show in the body.
Then there are mechanistic quibbles:
But that would cause cytoplasm to mix and result in the cells fusing, says Jennifer Lippincott-Schwartz, a cell biologist at the Howard Hughes Medical Institute Janelia Research Campus in Ashburn, Virginia.
Instead, she thinks that
membrane tubes may jut out and make minimal contact, just enough to
allow recipient cells to reach out and engulf the tube contents.
Chiara Zurzolo, a cell biologist at the Pasteur Institute in Paris, who has spotted prions and other neurodegenerative proteins travelling through nanotubes, says that many papers do not try to assess whether a tube is closed or open-ended, for example, or even whether the tubes allow the movement of vesicles or similar material.
The proliferation of tube types, and the different names for them, make coherent discussion difficult.
But getting clear images of living cells will always trump semantics, says Ian Smith, a cell biologist at the University of California, Irvine.
Most microscopy techniques can't get a clear view of these structures in action, even in cultured cells.
Smith is developing methods to visualize membrane nanotubes using lattice light-sheet microscopy, which monitors planes of light to build up 3D images.
He
hopes that the technique will be able to capture the process of
material transfer from one cell to another, from start to finish.
8 Smith admits that he's
taking a career risk: a colleague recently warned him this area was
'fringey'. But he takes this as a challenge.
At first people would tell him that the structures were artifacts or optical illusions, he recalls.
He likes that direction.
|