|
from QuantaMagazine Website
Spanish
version form tiny hollow tubular interconnections. In this video, payloads of useful molecules (inside blue circles) can be seen moving through these membranous nanotubes and microtubes to a cell in need of them. Such connections may help cancer cells
share their resistance to therapeutic drugs.
Click
image... and other bridges between cells act as conduits for sharing RNA, proteins or even whole organelles.
When the physician and scientist Emil Lou was an oncology fellow at Memorial Sloan Kettering Cancer Center about a decade ago, he was regularly troubled by the sight of something small but unidentifiable in his cancer-cell cultures.
Looking through the microscope, he said, he "kept finding these long, thin translucent lines," about 50 nanometers wide and 150 to 200 microns long, extending between cells in the culture.
He called on the world-class cell biologists in his building to explain these observations, but nobody was sure what they were looking at.
Finally, after delving into the literature, Lou realized that the lines matched what Hans-Hermann Gerdes' group at the University of Heidelberg had described as "nanotubular highways" or "tunneling nanotubes" (TNTs) in a 2004 paper (Nanotubular Highways for Intercellular Organelle Transport) in Science.
Lou worried that the lines he'd noticed might be illusory, so he checked the archive of tumor specimens from patients at the cancer center. Lo and behold, the same long cellular processes were present in the tumors, too, so he set out to investigate their relevance.
Since then, as a faculty member at the University of Minnesota, he's found evidence that tumor cells use these TNTs to share molecular messages in the form of short regulatory snippets of RNA called microRNA, enabling cancer cells resistant to chemotherapy drugs to confer the same resistance on their neighbors.
How did the tunneling nanotubes go unnoticed for such a long time?
Lou notes that in the last couple of decades, cancer research has centered primarily on detecting and therapeutically targeting mutations in cancer cells - and not the structures between them.
That's changing now. In the last few years, the number of researchers working on TNTs and figuring out what they do has risen steeply.
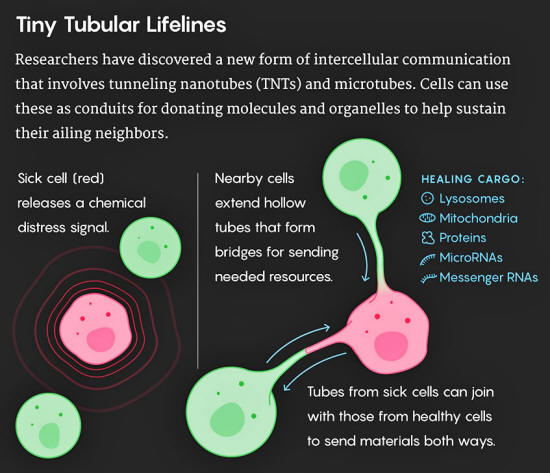
Research teams have discovered that TNTs transfer all kinds of cargo beyond microRNAs, including messenger RNAs, proteins, viruses and even whole organelles, such as lysosomes and mitochondria.
These fragile structures are appearing not only in the context of conditions such as cancer, AIDS and neurodegenerative diseases, but also in normal embryonic development.
Healthy adult cells don't usually make TNTs, but stressed or ailing cells appear to induce them by sending out signals to call for help.
It's unclear, though, how healthy cells sense that their neighbors need help or how they physiologically "know" what specific cargo to send.
Seeing Is Believing
The discovery of TNTs was a happy accident.
Amin Rustom, who was a member of Gerdes' group at the time and is still a researcher at the University of Heidelberg, recalls that it happened because he was using a new fluorescent dye to label cellular features of interest.
The protocol for using the dye called for several sequential washing steps, but he skipped some of them and took a look at his cells anyway. That's when he spotted the long tubular structures, which the dye had made more visible (he thinks the washing steps would have broken them).
With microscopy techniques, the group examined the structures further and determined that they are open channels through which organelles and membrane vesicles move from one cell to another.
At that point it became clear that the membrane tubes were,
It was not so easy, however, to convince others - some researchers suspected that these TNTs were experimental artifacts, not naturally occurring structures.
It took the group four or five years to publish their paper because of the strong skepticism with which the findings were met, he said. Confirming that TNTs are indeed an avenue for intercellular communication has continued to be a major challenge.
Cells have other options for exchanging molecules, most notably the structures called gap junctions and exosomes.
If TNTs are akin to skywalks, the enclosed footbridges that connect separate buildings, then gap junctions - gated pores that pass through the membranes of neighboring cells - are like doorways between adjacent rooms.
Exosomes, small vesicles shed by cells, were long thought to be cellular trash bags carrying debris, but scientists now recognize them as vehicles for carrying microRNAs and other signaling molecules between cells, sometimes over long distances.
The challenge in identifying the role of TNTs is that it's tricky to inhibit any one of these communication channels without interfering with the others.
Much recent effort also focuses on finding biomarkers for TNTs to distinguish them from similar-looking structures like filopodia, long cellular protrusions that are used for sensing and locomotion.
Filopodia are not open-ended, and they do not transfer cargo from one cell to another, but they can be hard to distinguish from TNTs based on shape alone.
What complicates matters is that TNTs appear in a wide variety of cell types and are morphologically diverse, showing up in a wide range of sizes.
In some cases they are large enough to be considered microtubes rather than nanotubes, and some researchers believe that the smaller TNTs are functionally different from microtubes. Efforts are ongoing to characterize the different subtypes of nano- and microtubes.
But many scientists still understandably harbor a lot of skepticism about the supposed functions of TNTs.
Chiara Zurzolo, the head of the department of cell biology and infection at the Pasteur Institute in Paris, does research on TNTs but acknowledges this difficulty.
Useful answers are emerging, however, because the budding field of TNT research is benefiting from advances in microscopy and other techniques.
Ian Smith, who studies TNTs at the University of California, Irvine, specializes in imaging methods such as lattice light-sheet microscopy, which is gentle enough to be used for observing live cells over hours or even days.
This method allows researchers to see the delicate structures of TNTs in living cells, and to track individual molecules moving between them.
Ian Smith, an associate professional researcher at the University of California, Irvine, uses gentle imaging techniques to observe tunneling nanotubes between living cells
for hours or days at a time.
Gal Haimovich, now a researcher at the Weizmann Institute of Science in Israel, joined Robert Singer's lab at the Albert Einstein College of Medicine as a postdoctoral fellow in 2012 with an interest in studying the intercellular transfer of RNA.
At the time, the standard model for RNA transfer was that the molecules were packaged into exosomes that then diffused between cells.
But that hadn't been directly observed; it had been inferred from biochemical experiments.
Haimovich wanted to use imaging methods developed in the Singer lab because they could visualize the RNA in transit and collect more quantitative data about it.
He grew two strains of cells - only one of which could express a particular mRNA - in the same culture dish and observed what happened.
Before his eyes, the mRNA molecules migrated through TNTs bridging the different cells.
To understand whether or not the cells actively regulate these transfers, Haimovich challenged them with heat shock and oxidative stress.
If changes in the environmental conditions changed the rate of RNA transfer, that,
He found that oxidative stress did induce an increase in the rate of transfer, while heat shock induced a decrease.
Moreover, this effect was seen if stress was inflicted on acceptor cells but not if it was also inflicted on donor cells prior to co-culture, Haimovich clarified by email.
His results (Intercellular mRNA Trafficking via Membrane Nanotube-like extensions in Mammalian Cells) were reported in the Proceedings of the National Academy of Sciences last year.
TNTs in Cancer
Cancer cells are often stressed - these rapidly dividing cells survive hypoxia, nutrient stress, oxidative stress and more.
So it's not surprising that they, like Haimovich's stressed cells, induce TNTs. Indeed, Lou's research shows that cells causing several types of cancer form between five and 100 times as many TNTs as normal healthy adult cells do.
Frank Winkler, a neurologist, oncologist and cancer researcher at the University of Heidelberg, discovered that the brain cancers called gliomas are full of tumor microtubes (TNTs' larger cousins).
He'd noticed these structures while watching single tumor cells grow in the brains of live mice, but he hadn't recognized their significance. The pathologist with whom he collaborated had attributed them to defects in the preparation of the specimens.
Not until Winkler and the pathologist saw these tiny tubes in living cells did they realize that the structures were real.
Winkler noticed that when he applied chemotherapy or radiation to patients' tumors, isolated cancer cells died, but those connected to each other through tumor microtubes and TNTs survived.
The networked cells, he said,
Lucy Reading-Ikkanda Quanta Magazine
In response to the stress of chemo or radiation therapy, the cancer cells form even more tumor microtubes and stronger networks, he explained.
Using network theory, he and his team are now working to decode the communication patterns among the linked cancer cells.
His working hypothesis is that there is a hierarchy of communication, and that certain cells - possibly those that have potent developmental properties like those of stem cells - act as "masterminds" of the network.
They may instruct other cells how to behave and drive both the progression of the tumor's growth and its resistance to therapies.
Winkler also found that the glioma tumor cells form connections with neighboring healthy cells - which opens up at least the possibility for some kind of two-way communication with the brain's tissues.
Michelle Monje, a pediatric oncologist at Stanford University, has shown that brain activity drives tumor progression in gliomas, and Winkler suspects that the tumor microtubes and TNTs might play a role in that.
But the good news is that these micro- and nanotubes also represent a completely new therapeutic target, Winkler said.
One approach is to try to develop drugs that will inhibit the formation of micro- and nanotubes to make the cancers more susceptible to chemo and radiation therapy. To that end, Winkler is currently working to characterize the diversity in size and function of these tiny tubes.
Another strategy is to exploit the tubular network to spread drugs:
Spotting a field brimming with opportunity, the pharmaceutical giant GlaxoSmithKline convened a meeting of TNT experts in September 2016 to explore the roles of TNTs - and how they could be disrupted or harnessed for therapeutic applications.
A New Bag of Tricks for Immune Cells
TNTs also play an important role in the immune system.
Their function involves dendritic cells, which Robbie Mailliard, an immunologist at the University of Pittsburgh, calls the,
Dendritic cells connect with each other via TNTs in a process called reticulation.
With his colleagues Giovanna Rappocciolo and Charles Rinaldo, Mailliard found that HIV and other viruses can exploit these TNTs to spread among dendritic cells.
Inhibition of the TNTs appears to inhibit the spread of HIV, Mailliard said.
Furthermore, the researchers found that those extremely rare people infected with HIV who are able to control the virus without any antiretroviral therapy (so-called "long-term nonprogressors") have a defect in their dendritic cells' ability to form TNTs.
This appears to be a genetic trait related to their cellular cholesterol levels. Now the researchers are investigating whether drugs that are commonly used to lower cholesterol levels could be repurposed to control viral infections by limiting reticulation.
HIV isn't the only infection that takes advantage of the dendritic cells' ability to form TNTs.
Prions (which are acquired, for instance, by eating beef from a cow with mad cow disease) and other misfolded proteins also hijack these cellular communication networks, Zurzolo explained.
Misfolded proteins that aggregate in the brain and catalyze the conversion of healthy proteins into misfolded ones are a major cause of neurodegenerative disease.
Examples of these misfolded proteins include tau, alpha-synuclein and huntingtin, which are central to the development of Alzheimer's disease, Parkinson's disease and Huntington's disease, respectively.
Until a few years ago, it wasn't clear how these infectious proteins spread from cell to cell, given that, unlike viruses or bacteria, misfolded proteins don't have an obvious way to get around.
Chiara Zurzolo has shown that cells infected with prions use tunneling nanotubes
to pass on the
infectious particles.
Zurzolo was studying prions at the Pasteur Institute in 2004 when Hans-Hermann Gerdes' group published its paper.
Another paper (Cutting Edge - Membrane Nanotubes Connect Immune Cells) published soon after by researchers at Imperial College London showed that cells of the immune system could connect via TNTs, and this led her to investigate whether prions could spread this way.
In 2009, Zurzolo published a study (Prions Hijack Tunnelling Nanotubes for Intercellular Spread) showing that dendritic cells could communicate with neurons through TNTs, and that when she infected a dendritic cell with a prion, the prion was transferred to the neurons.
This was the beginning of the idea that disease caused by prions could spread between cells through TNTs, she said.
The dendritic cells acquire the prion from the gut, which they constantly patrol for infection, Zurzolo said.
Then the motile dendritic cells circulate through the body and spread the prion via TNTs to the spleen and lymph nodes (which are immune system organs) and peripheral nerves. Once in the nervous system, the prion easily passes from one neuron to another through TNTs and thus reaches the brain.
Discovering how misfolded proteins move between cells,
In the past decade, she's shown that misfolded proteins involved in Alzheimer's and Parkinson's disease also spread from neuron to neuron through TNTs.
Still, a balance must be struck, because glial cells support neurons by communicating with them through TNTs, Rustom explained. Blocking the TNTs could therefore also interfere with the normal physiology of the brain.
Zurzolo has also refined her ideas about how and why the TNTs form.
Given the role of TNTs in the spread of cancer, HIV and neurodegenerative diseases, it's not surprising that researchers and pharmaceutical companies are interested in finding ways to disrupt them.
But what if these structures could also be harnessed for therapeutic purposes?
Unexpected Roles in Regenerative Medicine
Anne-Marie Rodriguez, a researcher at the Mondor Institute of Biomedical Research in Paris, has discovered that TNTs help injured heart cells recover from heart attacks.
After a heart attack, when cardiac muscle cells are injured by lack of oxygen, their damaged mitochondria release molecules called reactive oxygen species.
These molecules sound an alarm to nearby mesenchymal stem cells, which start producing more mitochondria and directing TNTs toward their ailing neighbors.
The stem cells then donate healthy replacement mitochondria through the TNT connections. In this way, Rodriguez explained, the stem cells are both sensors and rescuers of injured tissue.
Stephanie Cherqui, a regenerative medicine and stem cell researcher at the University of California, San Diego, fortuitously discovered a key role for TNTs in treatments she is developing for cystinosis, a rare disease caused by a single defective gene.
The defect causes the amino acid cystine to accumulate to toxic levels in tissues throughout the body, doing particular damage to the kidneys.
Cherqui's strategy to treat the disease, which she is developing in mouse models, is to extract hematopoietic (blood-producing) stem cells from the bones and insert a functional copy of the defective gene in them.
She can then clear the bone marrow of its original stem cells with chemotherapy and introduce the engineered stem cells so they can re-establish themselves.
How exactly did the engineered stem cells rescue the mouse?
First, they differentiated into immune cells called macrophages and traveled to the injured kidney tissues. Once there, the macrophages formed TNTs with injured cells and transferred lysosomes - tiny packages full of healthy enzymes - to the diseased cells, Cherqui explained.
The diseased cells also sent their defective lysosomes back to the macrophages through the same channels.
She is using a similar approach to treat a mitochondrial disease called Friedreich's ataxia.
In preliminary cell-culture studies, she showed that the engineered therapeutic stem cells can become macrophages that deliver healthy mitochondria through TNTs.
She is now studying the process in tissues including the brain, heart and muscle.
A Fundamental Function
While many scientists are enthusiastic about TNTs and their prospects for illuminating many aspects of health and disease, others remain skeptical because so much of the fundamental biology of TNTs is incompletely understood.
The researchers who work on them agree that there is an urgent need to understand the cellular signaling pathways that trigger the formation of these tiny tubes, to identify biomarkers that could be used to label them more distinctly, and to characterize the structural and functional diversity of nano- and microtubes.
Zurzolo agrees that rigorous scientific demonstrations of what these structures are and what they do will be needed to move the field forward.
Nevertheless, she is convinced that TNTs are important.
|