|
therapeutic payloads to precise locations in the body are the stuff of science fiction. But some researchers are trying to turn them into a clinical reality...
Chemotherapies
inevitably hit healthy bystander cells while blasting tumors,
sparking a slew of side effects. It is also a big ask for an
anticancer drug to find and destroy an entire tumor - some are
difficult to reach, or hard to penetrate once located.
These miniature machines could navigate directly to a tumor and smartly deploy a therapeutic payload right where it is needed.
Among his inspirations is the 1966 film Fantastic Voyage, in which a miniaturized submarine goes on a mission to remove a blood clot in a scientist's brain, piloted through the bloodstream by a similarly shrunken crew.
Although most of the film remains firmly in the realm
of science fiction, progress on miniature medical machines in the
past ten years has seen experiments move into animals for the first
time.
Some are moved and steered by outside forces, such as magnetic fields and ultrasound.
Others are driven by onboard chemical engines, and some are even built on top of bacteria and human cells to take advantage of those cells' inbuilt ability to get around.
Whatever the source of propulsion, it is hoped that these tiny robots will be able to deliver therapies to places that a drug alone might not be able to reach, such as into the centre of solid tumors.
However, even as
those working on medical nano- and microrobots begin to collaborate
more closely with clinicians, it is clear that the technology still
has a long way to go on its fantastic journey towards the clinic.
a miniaturized medical team goes on a mission to remove a blood clot in a scientist's brain. Contributor: Collection Christophel/Alamy Stock Photo
However, it is here that reality must immediately diverge from fiction.
Tiny robots would have
severe difficulty swimming against the flow of blood, he says.
Instead, they will initially be administered locally, then move
towards their targets over short distances.
Bots are therefore typically no more than 1-2 micrometers across.
However, most do not
fall below 300 nanometers. Beyond that size, it becomes more
challenging to detect and track them in biological media, as well as
more difficult to generate sufficient force to move them.
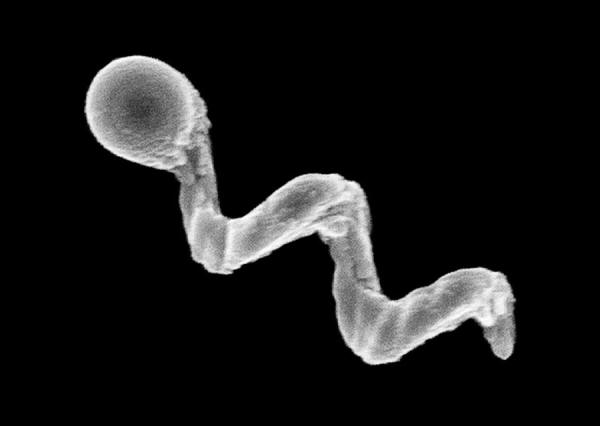
For instance, in 2009, Fischer - who was working at Harvard University in Cambridge, Massachusetts, at the time, alongside fellow nanoroboticist Ambarish Ghosh - devised a glass propeller, just 1-2 micrometers in length, that could be rotated by a magnetic field. 1
This allowed the structure to move through water, and by adjusting the magnetic field, it could be steered with micrometer precision.
In a 2018 study, 2 Fischer launched a swarm of micropropellers into a pig's eye in vitro, and had them travel over centimeter distances through the gel-like vitreous humor into the retina - a rare demonstration of propulsion through real tissue.
The swarm was able to slip through the network of biopolymers within the vitreous humor thanks in part to a silicone oil and fluorocarbon coating applied to each propeller.
Inspired by the slippery surface that the carnivorous pitcher plant
Nepenthes uses to catch insects, this minimized interactions between
the micropropellers and biopolymers. of a glass nanopropeller. Credit: Conny Miksch, MPI-IS
One group placed magnetic cores inside the membranes of red blood cells, 3 which also carried photo-reactive compounds and oxygen.
The cells' distinctive biconcave shape and greater density than other blood components allowed them to be propelled using ultrasonic energy, with an external magnetic field acting on the metallic core to provide steering.
Once the bots are in position,
light can excite the photosensitive compound, which transfers energy
to the oxygen and generates reactive oxygen species to damage cancer
cells.
Some of the most promising strategies aimed at treating solid tumors involve human cells and other single-celled organisms jazzed up with synthetic parts.
In Germany, a group led by Oliver Schmidt, a nano-scientist at Chemnitz University of Technology, has designed a biohybrid robot based on sperm cells. 4
These are some of the fastest motile cells, capable of hitting speeds of 5 millimeters per minute, Schmidt says. The hope is that these powerful swimmers can be harnessed to deliver drugs to tumors in the female reproductive tract, guided by magnetic fields.
Already, it has been shown that they can be magnetically guided to a
model tumor in a dish.
At the Chinese University of Hong Kong, meanwhile, nanoroboticist Li Zhang led the creation of microswimmers from Spirulina microalgae cloaked in the mineral magnetite.
The team then tracked a swarm of them inside rodent stomachs using magnetic resonance imaging. 5
The biohybrids were
shown to selectively target cancer cells. They also gradually
degrade, reducing unwanted toxicity.
Samuel Sánchez, a chemist at the Institute for Bioengineering of Catalonia in Barcelona, Spain, is developing nanomotors driven by chemical reactions for use in treating bladder cancer.
Some early devices relied on hydrogen peroxide as a fuel.
Its breakdown, promoted by platinum, generated water and oxygen gas bubbles for propulsion. But hydrogen peroxide is toxic to cells even in minuscule amounts, so Sánchez has transitioned towards safer materials.
His latest nanomotors are made up of honeycombed silica nanoparticles, tiny gold particles and the enzyme urease. 6
These 300-400-nm bots are driven forwards by the chemical breakdown of urea in the bladder into carbon dioxide and ammonia, and have been tested in the bladders of mice.
The bacterium activates the person's immune system, and is also the basis of the BCG vaccine for tuberculosis.
There is a need to improve on BCG in oncology, according to his collaborator, urologic oncologist Antoni Vilaseca at the Hospital Clinic of Barcelona.
Current treatments reduce recurrences and progression,
The nanobot approach that Sánchez is trying promises precision delivery.
He plans to insert his bots into the bladder (or intravenously), to motor towards the cancer with their cargo of therapeutic agents to target cancer cells, using abundant urea as a fuel.
He might use a magnetic field for guidance, if needed, but a more straightforward replacement of BCG with bots that do not require external control, perhaps using an antibody to bind a tumor marker, would please clinicians most.
showing a swarm of urease-powered nanomotors swimming in urea solution. Credit: Samuel Sánchez Ordóñez
The gut wall, for example, allows absorption of nutrients into the bloodstream, and offers an avenue for getting therapies into bodies.
However, a combination of cells, microbes and mucus stops many particles from accessing the rest of the body.
To deliver some
therapies, simply being in the intestine isn't enough - they also
need to be able to burrow through its defenses to reach the
bloodstream, and a nanomachine could help with this.
Their zinc-based nanomotor dissolved in the harsh stomach acids, producing hydrogen bubbles that rocketed the robot forwards.
In the lower gastrointestinal tract, they instead use magnesium.
In either
case, the metal micromotors are encapsulated in a coating that
dissolves at the right location, freeing the micromotor to propel
the bot into the mucous wall.
Fischer
envisages future micro- and nanorobots borrowing this approach to
deliver drugs through the gut.
Low oxygen levels force cells deep inside to switch to anaerobic metabolism and churn out lactic acid, creating acidic conditions.
As the oxygen gradient builds, the tumor becomes increasingly difficult to penetrate. Nanoparticle drugs lack a force with which to muscle through a tumor's fortifications, and typically less than 2% of them will make it inside8.
Proponents of nanomachines think that they can do better.
In nature, these Magnetococcus species seek regions that have low oxygen.
Martel has engineered such a bacterium to target active cancer cells deep inside tumors. 8.
The precise locations of low-oxygen regions are uncertain even with imaging, but once these bacteria reach the right location, their autonomous capability kicks in and they motor towards low-oxygen regions.
In a mouse, more than half the bacteria injected close to tumor grafts broke into this tumor region, each laden with dozens of drug-loaded liposomes.
Martel cautions,
however, that there is still some way to go before the technology is
proven safe and effective for treating people with cancer.
The motors navigate towards areas that are richer in DNA, such as tumor cells that undergoing apoptosis.
Once in place, the autonomous bots
seem to be picked up by cells more easily than passive particles are
- perhaps because the bots push against cells.
discusses a new computer interface with two members of his team. Credit: Caroline Perron
Fischer is similarly jaded by movie-inspired hype.
Nonetheless, advances in the past ten years have raised expectations of what is possible with current technology.
But having something wiggling under a microscope no longer has the same draw, without medical context.
With this in mind, many researchers creating nanorobots for medical purposes are working more closely with clinicians than ever before.
Neurologist Philipp Gruber, who works with stroke patients at Aarau Cantonal Hospital in Switzerland, began a collaboration with Nelson two years ago after contacting ETH Zurich.
The pair share an ambition to use steerable microbots to dissolve clots in people's brains after ischaemic stroke - either mechanically, or by delivering a drug.
Sánchez tells a similar story:
As these seedlings of clinical collaborations take root, it is likely that oncology applications will be the earliest movers - particularly those that resemble current treatments, such as infusing microbots instead of BCG into cancerous bladders.
But even
these therapeutic uses are probably at least 7-10 years away. In the
nearer term, there might be simpler tasks that nanobots can be used
to accomplish, according to those who follow the field closely.
The tiny gap between the metal implants and gum tissue is an ideal niche for bacterial biofilms to form, triggering infection and inflammation.
When this happens, the implant must often be removed, the area cleaned, and a new implant installed - an expensive and painful procedure.
He is collaborating with dental surgeon Karel
Klíma at Charles University in Prague.
A titanium oxide robot could be administered to implants using a syringe, then activated chemically or with light to generate active oxygen species to kill the bacteria.
Examples a few
micrometers in
length have so far been constructed, but much smaller bots - only a
few hundred nanometers in length - are the ultimate aim.
But the rising tide of in vivo experiments and the increasing involvement of clinicians suggests that microrobots might just be leaving port on their long journey towards the clinic.
References
|