|

by Bill Sardi and Timothy Hubbell
May 22, 2008
from
LewRockwell Website
|
Based in
Southern California, Bill Sardi is a noted and well-known
author, lecturer, speaker, and health researcher, with
numerous books and articles to his credit. He can be
reached at
BSardi@aol.com. Timothy Hubbell, a biochemist
from Cincinnati, first called attention to this
discovery and provided consultation on the biochemistry.
Bill Sardi
is author of the new book:
You Don't Have To Be Afraid Of
Cancer Anymore |
It works 100% of the
time to eradicate cancer completely, and cancer does not recur even
years later. That is how researchers describe the most convincing
cancer cure ever announced.
The weekly injection of just 100 billionths of a gram of a harmless
glyco-protein (a naturally-produced molecule with a sugar component
and a protein component) activates the human immune system and
cures
cancer for good, according to human studies among breast cancer and
colon cancer patients, producing complete remissions lasting 4 and 7
years respectively.
This glyco-protein cure
is totally without side effect but currently goes unused by cancer
doctors.
Normal Gc protein (also called Vitamin-D binding protein), an
abundant glyco-protein found in human blood serum, becomes the
molecular switch to activate macrophages when it is converted to its
active form, called Gc
macrophage activating factor (Gc-MAF). Gc
protein is normally activated by conversion to Gc-MAF with the help
of the B and T cells (bone marrow-made and thymus gland-made white
blood cells).
But, as researchers
explain it themselves, cancer cells secrete an enzyme known as
alpha-N-acetylgalactosaminidase (also called Nagalase) that
completely blocks conversion of Gc protein to Gc-MAF, preventing
tumor-cell killing by the macrophages. This is the way cancer cells
escape detection and destruction, by disengaging the human immune
system. This also leaves cancer patients prone to infections and
many then succumb to pneumonia or other infections.
The once-weekly injection of minute amounts of Gc-MAF, just 100
nanograms (billionths of a gram), activates macrophages and allows
the immune system to pursue cancer cells with vigor, sufficient to
produce total long-term cures in humans.
Nobuto Yamamoto, director of the Division of Cancer
Immunology and Molecular Biology, Socrates Institute for Therapeutic
Immunology, Philadelphia, Pennsylvania, says this is,
“probably
the most potent macrophage activating factor ever discovered.”
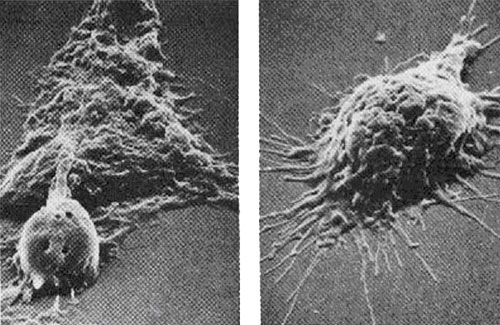
A MACROPHAGE
OVERCOMES AND EATS A CANCER CELL
FROM THE UPJOHN COMPANY, THE IMMUNE SYSTEM
A MACROPHAGE
OVERCOMES AND EATS A CANCER CELL
FROM THE UPJOHN
COMPANY, THE IMMUNE SYSTEM
Once a sufficient number of activated macrophages are produced,
another Gc-MAF injection is not needed for a week because
macrophages have a half-life of about six days.
After 16-22 weekly doses
of Gc-MAF the amount of Nagalase enzyme fell to levels found in
healthy people, which serves as evidence tumors have been completely
eliminated. The treatment was fool-proof - it worked in 100% of
16 breast cancer patients and there were no recurrent tumors over a
period of 4 years, says a report in the January 15 issue of the
International Journal of Cancer. [International Journal Cancer.2008
January15; 122(2):461-7]
In another startling follow-up report by Dr. Yamamoto and
colleagues, published in the upcoming July issue of Cancer
Immunology Immunotherapy, Gc-MAF therapy totally abolished tumors in
8 colon cancer patients who had already undergone surgery but still
exhibited circulating cancer cells (metastases).
After 32-50 weekly
injections,
”all colorectal cancer patients exhibited healthy
control levels of the serum Nagalase activity, indicating
eradication of metastatic tumor cells,” said researchers, an effect
that lasted 7 years with no indication of cancer recurrence either
by enzyme activity or CT scans, said researchers.
[Cancer
Immunology, Immunotherapy Volume 57, Number 7 / July 2008]
Published
in an early online edition of this journal, this confirming report
has received no attention by the new media so far, despite its
striking importance.
Gc-MAF treatment for cancer has been agonizingly slow to develop.
Dr. Yamamoto first described this immuno-therapy in 1993. [The
Journal of Immunology, 1993 151 (5); 2794-2802]
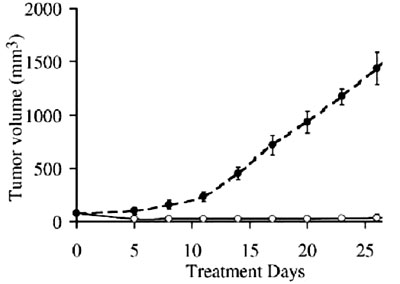
Untreated mice ○ Mice
given macrophage activating factor
In a similar animal experiment published in 2003, researchers in
Germany, Japan and the United States collaborated to successfully
demonstrate that after they had injected macrophage activating
factor (Gc-MAF) into tumor-bearing mice, it totally eradicated
tumors. [Neoplasia 2003 January; 5(1): 32–40]
In 1997 Dr. Yamamoto injected GcMAF protein into tumor-bearing mice,
with the same startling results. A single enzyme injection doubled
the survival of these mice and just four enzyme injections increased
survival by 6-fold. [Cancer Research 1997 Jun 1; 57(11):2187-92]
In 1996 Dr. Yamamoto reported that all 52 cancer patients he had
studied carried elevated blood plasma levels of the immune
inactivating alpha-N-acetylgalactosaminidase enzyme (Nagalase),
whereas healthy humans had very low levels of this enzyme. [Cancer
Research 1996 Jun 15; 56(12):2827-31]
In the early 1990s, Dr. Yamamoto first described how the human
immune system is disengaged by enzymes secreted from cancer cells,
even filing a patent on the proposed therapy. [US Patent 5326749,
July 1994; Cancer Research 1996 June 15; 56: 2827-31]
Activated Gc protein has been used in humans at much higher doses
without side effect. This Gc macrophage activating factor (Gc-MAF)
has been shown to be effective against a variety of cancers
including breast, prostate, stomach, liver, lung, uterus, ovary,
brain, skin, head/neck cancer, and leukemia.
Although GcMAF is also called Vitamin-D binding protein, the
activation of macrophages does not require Vitamin D.
It cannot be said the Gc-MAF cancer cure has gone unheralded.
Reuters News covered this developing story in January. But the news
story still did not receive top billing nor did it fully elucidate
the importance of the discovery, actually made years ago, that the
human body is capable of abolishing cancer once its immune system is
properly activated.
Gc-MAF is a naturally made molecule and is not patentable, though
its
manufacturing process is patent protected.
There is no evidence of
any current effort to commercialize this therapy or put it into
practice. Should such an effective treatment for cancer come into
common practice, the income stream from health-insurance plans for
every oncology office and cancer center in the world, would likely be
reduced to the point of financial insolvency and hundreds of
thousands of jobs would be eliminated.
The National Cancer Institute estimates cancer care in the U.S.
costs ~$72 billion annually (2004). Furthermore, about $55 billion
of cancer drugs are used annually, none which have not significantly
improved survival rates throughout the history of their use.
If a typical cancer
patient had to undergo 30 GcMAF injections at a cost of $150 per
injection, that would cost ~$4500, not counting doctor’s office
visits and follow-up testing. For comparison, gene-targeted cancer
drugs range from $13,000 to $100,000 in cost per year and produce
only marginal improvements in survival (weeks to months). [Targeted
Oncology 2007 April, 2 (2); 113-19]
Up to this point, the National Cancer Institute is totally silent on
this discovery and there is no evidence the cancer care industry
plans to quickly mobilize to use this otherwise harmless treatment.
Addendum
Sadly, the treatment
you have just read about is not available anywhere. Its inventor
is attempting to patent a version of it to profiteer off of it
even though there is no need to improve upon the GcMAF molecule
- it worked without failure to completely cure four different
types of cancer with no long-term remissions and without side
effect.
While GcMAF is
produced by every healthy adult, there are no centers available
to extract it from blood samples and inject it into patients
with malignancies. Hopefully, someday, doctors will write
protocols to do this and submit them to institutional review
boards so GcMAF treatment can be performed on an experimental
basis.
GcMAF is a
naturally-made molecule that cannot be patented.
This article was
written to reveal that there are proven cancer cures that go
unused. Of interest, not one oncologist has requested
information about GcMAF since this article was written, while I
have been barraged with inquires from cancer patients, their
families and some interested physicians who are not cancer
doctors.
-Bill Sardi
Help NHF get the word out about GcMAF and other proven cures for
cancer that are being ignored. Learn how NHF is the leading health
freedom organization, for example, battling for your right to
maintain access to dietary supplements without restrictions imposed
by quasi-governing bodies like
CODEX and our other missions.
Search the NHF website
for more helpful information and become a member by
clicking here.
Macrophage Activation May Suppress Breast
Cancer Metastasis
by David
Douglas
NEW YORK
February 20, 2008
Reuters Health
Vitamin D-binding
protein-derived macrophage activating factor (Gc-MAF)
appears to be an effective immunotherapeutic agent in patients
with metastatic breast cancer, according to US and Japanese
researchers.
"Serum vitamin
D-binding protein – known as Gc protein – is the
precursor of the principal macrophage activating factor,"
lead investigator Dr. Nobuto Yamamoto told Reuters
Health.
"Treatment of purified Gc protein with beta-galactosidase
and sialidase generates Gc-MAF," he added, "the most potent
macrophage activating factor ever discovered, which produces
no side effect in humans."
Dr. Yamamoto of the
Socrates Institute for Therapeutic Immunology,
Philadelphia and colleagues note that in vitro studies show that
macrophages treated with Gc-MAF have a highly tumoricidal effect
in mammary adenocarcinomas.
To investigate whether the approach can be effective in humans,
the researchers studied 16 non-anemic breast cancer patients who
were given,
"a minute amount
– 100 nanograms per week – of Gc-MAF," Dr. Yamamoto said.
The researchers
found that after 16 to 22 Gc-MAF doses, initially elevated
nagalase levels, which reflect the tumor burden, fell to those
found in healthy controls. Follow-up over 4 years showed that
the level remained low and that there was no tumor recurrence,
they report in the January 15th issue of The International
Journal of Cancer.
The findings, the team concludes, clearly demonstrate
"the
importance of focusing cancer immunotherapy on
macrophage activation."
International Journal Cancer
2008 Jan 15;
122(2):461-7
Immunotherapy of metastatic breast cancer patients with vitamin
D-binding protein-derived macrophage activating factor (Gc-MAF).
Yamamoto N,
Suyama H,
Yamamoto N,
Ushijima N.
Division
of Cancer Immunology and Molecular Biology
Socrates
Institute for Therapeutic Immunology
Philadelphia, PA
19126-3305, USA.
nobutoyama@verizon.net
Serum vitamin
D3-binding protein (Gc protein) is the precursor for the
principal macrophage activating factor (MAF). The MAF precursor
activity of serum Gc protein of breast cancer patients was lost
or reduced because Gc protein was deglycosylated by serum
alpha-N-acetylgalactosaminidase (Nagalase) secreted from
cancerous cells. Patient serum Nagalase activity is proportional
to tumor burden.
The deglycosylated
Gc protein cannot be converted to MAF, resulting in no
macrophage activation and immunosuppression. Stepwise incubation
of purified Gc protein with immobilized beta-galactosidase and
sialidase generated probably the most potent macrophage
activating factor (termed Gc-MAF) ever discovered, which
produces no adverse effect in humans.
Macrophages treated
in vitro with Gc-MAF (100 pg/ml) are highly tumoricidal to
mammary adenocarcinomas. Efficacy of Gc-MAF for treatment of
metastatic breast cancer was investigated with 16 nonanemic
patients who received weekly administration of Gc-MAF (100 ng).
As Gc-MAF therapy progresses, the MAF precursor activity of
patient Gc protein increased with a concomitant decrease in
serum Nagalase.
Because of
proportionality of serum Nagalase activity to tumor burden, the
time course progress of Gc-MAF therapy was assessed by serum
Nagalase activity as a prognostic index. These patients had the
initial Nagalase activities ranging from 2.32 to 6.28 nmole/min/mg
protein. After about 16-22 administrations (approximately 3.5-5
months) of Gc-MAF, these patients had insignificantly low serum
enzyme levels equivalent to healthy control enzyme levels,
ranging from 0.38 to 0.63 nmole/min/mg protein, indicating
eradication of the tumors.
This therapeutic
procedure resulted in no recurrence for more than 4 years.
Cancer Immunology, Immunotherapy
2008 July 57 (7):
online
Immunotherapy of metastatic colorectal cancer with vitamin
D-binding protein-derived macrophage-activating factor, Gc-MAF
Nobuto
Yamamoto
Hirofumi Suyama
Hiroaki Nakazato
Nobuyuki Yamamoto
Yoshihiko Koga
Abstract
Serum vitamin D
binding protein (Gc protein) is the precursor for the principal
macrophage-activating factor (MAF). The MAF precursor activity
of serum Gc protein of colorectal cancer patients was lost or
reduced because Gc protein is deglycosylated by serum α-N-acetylgalactosaminidase
(Nagalase) secreted from cancerous cells.
Deglycosylated Gc
protein cannot be converted to MAF, leading to immunosuppression.
Stepwise treatment of purified Gc protein with immobilized β-galactosidase
and sialidase generated the most potent macrophage-activating
factor (Gc-MAF) ever discovered, but it produces no side effect
in humans. Macrophages treated with Gc-MAF (100 pg/ml) develop
an enormous variation of receptors and are highly tumoricidal to
a variety of cancers indiscriminately.
Administration of
100 nanogram (ng)/human maximally activates systemic macrophages
that can kill cancerous cells. Since the half-life of the
activated macrophages is approximately 6 days, 100 ng Gc-MAF was
administered weekly to eight nonanemic colorectal cancer
patients who had previously received tumor-resection but still
carried significant amounts of metastatic tumor cells.
As Gc-MAF therapy
progressed, the MAF precursor activities of all patients
increased and conversely their serum Nagalase activities
decreased. Since serum Nagalase is proportional to tumor burden,
serum Nagalase activity was used as a prognostic index for time
course analysis of Gc-MAF therapy.
After 32–50 weekly
administrations of 100 ng Gc-MAF, all colorectal cancer patients
exhibited healthy control levels of the serum Nagalase activity,
indicating eradication of metastatic tumor cells.
During 7 years after
the completion of Gc-MAF therapy, their serum Nagalase activity
did not increase, indicating no recurrence of cancer, which was
also supported by the annual CT scans of these patients.
|


