|

by Mark Sircus
Director
15 November 2011
from
IMVA Website
There is growing
evidence that Americans would have better health
and a lower incidence of cancer and fibrocystic disease of the
breast if
they consumed more iodine. A decrease in iodine intake coupled with
an increased consumption of competing halogens, fluoride and
bromide, has created an epidemic of iodine deficiency in America.
Dr. Donald Miller, Jr.
The toxicity of modern
life is impacting iodine levels and in the countries that fluoridate
their water this impact is maximized.
It is well known that
the toxic halides:
fluoride and bromide, having structure similar to
iodine, can competitively inhibit iodine absorption and binding in
the body.
All the halogens use the same receptors in the body so
fluoride’s danger for people is centered in great part on this fact.
Americans and Brazilians, who are more exposed to
fluoride than other populations, have a desperate need for more
iodine. Taking iodine in its
nascent form is not only the best way
to increase iodine levels in the safest and most effective way
possible for adults and children whose thyroids are already
compromised, but it will also greatly aid in ridding the body of
dangerous fluoride, bromide, chlorine, perchlorates and heavy
metals.
In our age of increasing radioactivity and toxic poisoning
specifically with fluoride,[1] chlorine, bromide, and
even mercury, iodine is a necessary mineral.
Iodine is extremely important since the cells need it to regulate
their metabolism. Without it, people are known to suffer from
swollen glands in the throat, thyroid diseases, increased fluoride
toxicity, decreased fertility rates, increased infant mortality
rates, and (with severe deficiency) mental retardation.
It has been theorized
that iodine deficiency is a causal factor of
ADHD in babies of
iodine-deficient mothers.
Iodine intake
immediately increases the excretion of bromide, fluoride,
and some heavy metals including mercury and lead. Bromide and
fluoride are not removed by any other chelator or detoxifying
technique.
Dr. Kenezy Gyula
Korhaz states that iodine
chelates heavy metals such as mercury,
lead, cadmium, aluminum, and halogens such as fluoride and bromide,
thus decreasing their iodine-inhibiting effects,[2]
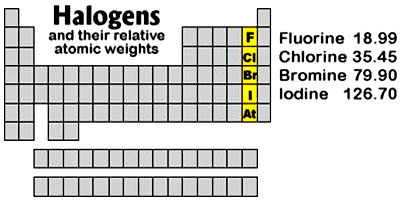
especially of the halogens. Iodine has the highest atomic weight of
all the common halogens (126.9).
Iodine is the only
option when it comes to removing these toxic haloids from the
thyroid and even the pineal gland where fluoride concentrates,
especially when there is a deficiency of iodine in the body.
The human pineal gland
contains the highest concentration of
fluoride in the body. Fluoride is associated with depressed pineal
melatonin synthesis and this depression increases one’s chance of
cancer.
Dr. David Brownstein
says that fluoride inhibits the ability of the thyroid gland to
concentrate iodine and research has shown that fluoride is much more
toxic to the body when there is iodine deficiency present.
Brownstein says that
after only one dose of iodine, the excretion of fluoride increases
by 78%.[3]
On January 7, 2011, the US Department of Health & Human Services
(HHS) proposed lowering the recommended level used in the water
fluoridation program to 0.7 ppm, because of the very high incidence
of
dental fluorosis among American children.
An amazing 41% of ALL
American children aged 12-15 are now impacted by this condition.
Sodium fluoride is
commonly used as a rat poison.
Globalists and eugenicists have decided to add it to
water supplies with the message to the public that it
is good for teeth, despite warnings from the ADA stating
that young children risk a disease called dental fluorosis.
After hailing water
fluoridation as one of the 10
'greatest health achievements' of the 20th
Century (says
the CDC), the government is calling for a
reduction in the
amount of fluoride it adds to public water supplies, citing its
negative effect on teeth when promotion of healthy teeth is the
basic reason given for adding fluoride to the water.
An
August 2006 Chinese study found that fluoride in drinking water
damages children’s liver and kidney functions. One of the strongest
physiological effects of fluorides in drinking water (e.g. hydrofluorosilicic acid) is on the kidney, a point to consider in
light of increased rates of kidney failure during recent decades.[4]
Kidney disease
markedly increases an individual’s susceptibility to
fluoride toxicity. In healthy adults, the kidneys are able to
excrete approximately 50% of an ingested dose of fluoride.
However, in adults with
kidney disease, the kidneys may excrete as little as 10-20%, and
young children may only excrete 15% of an ingested dose - thus
increasing the body burden of fluoride and increasing an
individual’s susceptibility to fluoride poisoning (e.g.
renal osteodystrophy).
Scientific evidence over
the past 50 plus years has shown that
sodium fluoride shortens our life span, promotes various cancers
and mental disturbances, and most importantly, makes humans
stupid, docile, and subservient, all in one neat little package.
A
Scientific American
study,
“concluded that fluoride can subtly alter endocrine function,
especially in the thyroid.”
The National Research
Council of the National Academies in a 2006 report on page 266
said,
“In summary,
evidence of several types indicates that fluoride affects normal
endocrine function or response; the effects of the
fluoride-induced changes vary in degree and kind in different
individuals. Fluoride is therefore an endocrine disruptor in the
broad sense of altering normal endocrine function.”
Halogen
Displacement
The mechanism behind “halogen displacement” was probably best
described by J. C. Jarvis, M.D. (Folk Medicine, 1958, HB, p. 136), who wrote:
“The clinical
activity of any one of these four halogens is in inverse
proportion to its atomic weight. This means that any one of the
four can displace the element with a higher atomic weight, but
cannot displace an element with a lower atomic weight.
For example,
fluorine can displace chlorine, bromine, and iodine because
fluorine has a lower atomic weight than the other three.
Similarly, chlorine can displace bromine and iodine because they
both have a higher atomic weight.”
Likewise, bromine can
displace iodine from the body because iodine has a higher atomic
weight.
A reverse order is not
possible.
European doctors used
fluoride as a
thyroid-suppressing medication
for patients with HYPER-thyroidism (over-active thyroid).
Fluoride was utilized because it was found to be effective at
reducing
the activity of the thyroid gland - even at doses as low as 2
mg/day.
The regular use of
iodine will go a very long way toward mitigating the damages done in
our bodies by the fluoride that we are exposed to.
The Nascent form is the
easiest way of increasing iodine levels for adults and children
whose thyroids are already compromised by fluoride. Nascent Iodine
is the atomic form and it is special. Nascent has the advantage of
being in the I¹ atomic (as opposed to I2 or I3 molecular forms) form
meaning that there is no digestion, no breaking down of molecules of
iodine.
It is already broken
down through an electromagnetic process.
For heavy lifting when needing iodine in large quantities for
transdermal application (painting the breasts daily with iodine for
breast cancer is one example) I recommend the age old
Lugol’s
formula.
Liquid forms of minerals always seem better than solid pill
forms because the body has an easier time digesting and absorbing
liquid forms with the nascent form ready to be utilized instantly in
any way the body needs.
Some tissues utilize
iodide and others iodine and the thyroid always needs atomic iodine
to make T3 and T4 metabolic thyroid hormones.
References
[1] Fluoride is
associated with cancer and it also accumulates in the thyroid as
well as the pineal gland, an important hormone control center.
Dr. Jennifer Luke found out that the pineal gland which produces
serotonin and melatonin was also a calcifying tissue, like the
teeth and the bones, so she hypothesized it would concentrate
fluoride to very high levels. Luke had 11 cadavers analyzed in
the UK and found very high levels of fluoride in the calcium
hydroxy apatite crystals produced by the gland. The average was
9000 ppm and went as high as 21,000 in one case. These levels
are equal to or higher than fluoride levels in the bones of
people suffering from skeletal fluorosis. Luke hypothesizes that
one of the four enzymes needed to convert the amino acid
tryptophan (from the diet) into melatonin is being inhibited by
fluoride. Melatonin is responsible for regulating all kinds of
activities including the onset of puberty. It is thought that
the fall of melatonin levels acts like a biological clock and
triggers the onset of puberty. In her gerbil study she found
that the high fluoride treated animals were reaching puberty
earlier than the low fluoride ones. Considering the seriousness
of a possible interference by fluoride on a growing child’s
pineal gland (and for that matter, elderly pineal glands)
underlines the need for higher iodine intake to increase
fluoride elimination.
[2] Sticht, G., Käferstein, H., Bromine. In Handbook on Toxicity
of Inorganic Compounds - Seiler HG and Sigel, H Editors, Marcel
Dekker Inc, 143-151, 1988.
[3] David Brownstein, Iodine, Why You Need It, Why You Can’t
Live Without It; https://www.drbrownstein.com/bookstore_Iodine.php
[4]
http://fluoride-class-action.com/wp-content/uploads/carol-clinch-2009-fluoride-and-kidneys.pdf
|

