Abstract
The present invention relates to medically efficacious agents
coated with substance that forms a liquid impermeable but gas
permeable layer, the treatment of medical conditions therewith,
and particularly medical conditions at least partially
characterized by blockage or other malfunction of ducts of
exocrine glands and especially ducts of sweat glands.
Claims
(OCR
text may contain errors)
-
The
use of sodium chloride formulated such that it cannot
cross epithelial barriers in the manufacture of a
medicament for the treatment of medical conditions at
least partially characterised by blockage or other
malfunction of exocrine glands.
-
The
use according to claim 1 wherein the sodium chloride is
in crystal form.
-
The
use according to claims 1 or 2 wherein the sodium
chloride is coated with an agent that forms a liquid
impermeable but gas permeable layer.
-
The
use according to claim 3 wherein the agent is a ceramic,
a polymer or a natural wax.
-
The
use according to claim 3 wherein the agent encapsulates
sodium chloride to foπn a sphere.
-
The
use according to claim 5 wherein the sphere is of a
diameter between 1 mm and 10 mm.
-
The
use according to claims 5 or 6 wherein the sphere
comprises sodium chloride crystals coated with beeswax
hardened with cornstarch and talc.
-
Sodium chloride coated with an agent that forms a liquid
impermeable but gas permeable layer.
-
The
use according to claim 8 wherein the agent is a ceramic,
a polymer or a natural wax.
-
The
use according to claim 8 wherein the agent encapsulates
sodium chloride to form a sphere.
-
The
use according to claim 10 wherein the sphere is of a
diameter between 1 mm and 10 mm.
-
The
use according to either of claims 10 or 11 wherein the
sphere comprises sodium chloride crystals coated with
beeswax hardened with cornstarch and talc.
-
A
patch suitable for adherence to skin containing sodium
chloride adapted for use in the treatment of medical
conditions at least partially characterized by the
blockage or other malfunction of exocrine glands.
-
The
patch according to claim 13 comprising a sticking
plaster suitable for adherence to skin.
-
The
patch according to claim 14 comprising a hypoallergenic
water resistant plaster.
-
The
patch according to either of claims 14 or 15 in the form
of a figure eight.
-
The
patch according to claim 16 further comprising two
spherical granules of sodium chloride.
-
A
patch according to any of claims 13 to 17 further
comprising coated sodium chloride according to any of
claims 8-12.
-
A
device consisting of a holder adapted for holding a
medically efficacious compound, an energy source and an
actuator driven by the energy source for temporarily and
at intervals placing the compound against the skin of a
subject.
-
The
device according to claim 19 adapted to be worn around
the abdomen or thorax of a subject.
-
The
device according to either of claims 19 or 20 wherein
the energy source is a low voltage battery.
-
The
device according to any of claims 19-21 further
comprises an electronic timer.
-
The
device according to any of claims 19-22 wherein the
actuator is a spring return push-rod solenoid.
-
A
device according to any of claims 19-23 adapted to hold
sodium chloride.
-
A
device according to any of claims 19 to 23 adapted to
hold sodium chloride coated according to any of claims
8-12.
-
A
preparation comprising a medically efficacious substance
coated or otherwise enclosed by an agent that forms a
liquid impermeable but gas permeable layer for use as a
medicament.
-
A
preparation according to claim 26, said agent formulated
such that said substance cannot cross epithelial
barriers in mammals.
-
A
preparation according to claim 26, said agent formulated
such that said substance cannot cross membrane barriers
in mammals.
-
A
preparation according to claim 26, said agent formulated
such that said substance cannot cross into cells of
mammals.
-
A
preparation according to any of claims 26-29, in a form
selected from the group consisting of: a pill, a tablet,
a lozenge, a bolus, a capsule, a caplet, a granule, a
nanoparticle, and a microparticle.
-
A
preparation according to claim 30, being in granular,
nanoparticle or microparticle form selected from the
group consisting of: a suspension, a cream, and a paste.
-
A
preparation according to any of claims 26-29, prepared
for use with a patch for holding said preparation near
to or against the skin of a patient.
-
A
preparation according to any of claims 26-29, prepared
for implantation into the body of a patient.
-
A
preparation according to any of claims 26-33, wherein
said agent is selected from the group consisting of: a
ceramic, a polymer, a natural wax, and beeswax hardened
with cornstarch and talc.
-
A
preparation according to any of claims 26-34, wherein
said substance is selected from the group consisting of:
sodium chloride, capsaicin, metformin, salicylic acid,
and a derivative of salicylic acid.
-
A
preparation according to any of claims 26-34, wherein
said substance is selected from the group consisting of:
a substance endogenous to the body, a food substance, a
plant material, and a drag.
-
A
preparation according to any of claims 26-36, combined
with a preparation of at least one ingredient designed
for delivery into solution.
-
A
preparation according to any of claims 26-36 combined
with a preparation of at least one ingredient designed
for delivery into solution for a therapeutic purpose.
-
A
method of manufacture of a medicament comprising coating
or otherwise enclosing a medically efficacious substance
in an agent that forms a liquid impermeable but gas
permeable layer.
Description
(OCR
text may contain errors)
COMPOSITIONS COMPRISING COMPONENTS COATED
WITH A
LIQUID IMPERMEABLE BUT GAS PERMEABLE LAYER,
USE THEREOF
FOR TREATING CUTANEOUS
AND OTHER
EXOCRINE GLAND DISEASES
The present
invention relates to the treatment of medical conditions and
particularly medical conditions at least partially characterized
by blockage or other malfunction of exocrine glands,
particularly ducts of exocrine glands, and especially ducts of
sweat glands.
People in advanced western societies such as the UK and USA are
increasingly likely to suffer from a number of chronic diseases
(e.g. essential hypertension, asthma, inflammation of the
gastro-intestinal tract) and despite expensive medical
intervention the incidence of such diseases continue to rise.
Several of these chronic conditions are known to be related to
the blockage of ducts of exocrine glands. For example, it is
well known that the blockage of sweat gland ducts and retained
sweat can lead to Miliaria, an acute inflammatory skin condition
better known as Prickly Heat.
Adult humans are estimated to have between 2 and 4 million sweat
glands, with ducts to the skin surface. Sweat is known to be a
fluid consisting mainly of water, with waste products such as
urea, plus sodium and other salts.
Miliaria occurs when sweat gland ducts are obstructed. As a
consequence, -sweat does not reach the skin surface and is
trapped in the epidermis or dermis, where it causes a prickling
sensation often accompanied by severe itching.
Even when
ducts are blocked, the sweat glands continue to output fluid and
just below the position of the blockage, the pressure of the
sweat ruptures the duct and forces sweat into the surrounding
skin. If sweat increases as a result of emotion, heat or
exercise, then the amount of damage to the surrounding skin may
be even greater.
Depending on the depth in which the obstruction occurs,
different types of lesions appear.
Ductal
obstruction in the uppermost epidermis results in Miliaria
crystallina with asymptomatic superficial vesicles, whilst
obstruction with inflammation occurring deeper in the epidermis
leads to Miliaria rubra, which is characterised by red lesions
and appears as pruritic and tender red macules or papules.
This type
of Miliaria can become infected and pustular and is very
unpleasant. Current treatment consists of remaining in a cool
environment for some weeks, and the topical application of pure
lanolin, which has a temporary effect.
If duct blockage occurs in the upper dermis, in a layer richly
provided with nerve endings (the itch layer), then there is
painful and pruritic inflammation. This is a previously unknown
Miliaria type identified by the inventor (designated Miliaria
type 3), and which is believed to leads to atopic dermatitis or
eczema.
In the deepest and most severe form of Miliaria, called Miliaria
profunda, ductal obstruction occurs near the entrance of the
duct into the dermal papillae resulting in subtle asymptomatic,
flesh colored papules. In Miliaria profunda the sweat spreads
into the surrounding skin and, unseen and unnoticed, ruptures
adjacent blood capillaries.
The
inventor believes this to be a potential cause of essential
hypertension, as more and more ruptured capillaries cause
pressure in the blood circulation to rise, generally continuing
to rise with age. The rupture of the capillaries may sometimes
trigger the formation of dangerous thrombi, particularly in the
lower legs.
Persons who are unacclimatised to heat but who remain in high
ambient temperatures, or who exert themselves in high
temperatures, are likely to suffer extensive acute Miliaria
profunda. The blockage of many gland ducts disables the ability
of the body to cool properly by sweat evaporation, potentially
leading to heat exhaustion or heat stroke.
Miliaria can also occur where exercise is undertaken or there is
heat exposure in persons wearing occlusive clothing. This is a
common cause of various types of Miliaria for example in
occupations such as mining, fire fighting, catering and other
physical jobs in hot conditions.
Human facial skin is thin, being only about 10% of the thickness
of skin on the back. Blockages in the sweat ducts of facial skin
may result in any type of skin Miliaria, and the inventor found
that a common type is a combination of Miliaria rubra and
Miliaria type 3 (see above).
This
combination Miliaria is often temporary but may eventually cause
chronic erythema, flushing, and blushing with cyclical crops of
pustules and papules. Ultimately, particularly in men, this can
lead to distinctive tissue hyperplasia and disfiguring phyma,
such as rhinophyma, a bulbous hypertrophy of the nose.
The
inventor believes this combination of Miliaria types may also
result in rosacea featuring red lines, or telangiectasia, said
to be characteristic thereof. Blockages of the sebaceous
exocrine ducts of facial and other skin causes the common
condition of acne vulgaris which often accompanies roseacea.
Furthermore, the inventor has found that, particularly in
females, blockage of exocrine ducts may cause sweat filled
vacuoles in the dermis. If the affected area of skin is
subjected to pressure, for example the sitting contact area of
the thighs and the buttocks, adipose tissue is extruded through
the connective tissue of the border into the dermis to produce a
characteristic irregular dimpled effect, better known as
cellulite.
The
inventor has found that the disease of psoriasis may be a type
of Miliaria where as a result of trauma to the skin a microbial
infection becomes trapped beneath sweat duct blockages.
The inventor has also recognised that many other diseases are
affected by the blockage or other malfunction of exocrine ducts
in tissues other than the skin.
The inventor believes that the blockage of exocrine ducts on the
head may cause constriction or interruption of blood circulation
to the brain which itself may result in migraine. Furthermore,
such chronic blockage may possibly lead to various types of
neurodegeneration.
The inventor has established that blockage of exocrine glands in
the mucosal surfaces of the lungs contributes to asthma. The
inventor also believes that allergic rhinitis or hay fever is
caused by a blockage of exocrine glands of the mucosal surface
of the interior of the nose.
Any blockage of exocrine glands may affect the skin of the
breasts, which are well supplied with sweat glands and ducts.
Also, female breasts have some degree of fluid secretion
activity from milk glands throughout adult life, even when
apparently not lactating. Additional milky discharge can also be
due to drugs or hormones which stimulate milk production, or to
mechanical stimulation of the nipple.
The
inventor believes that milk gland ducts are subject to blockage
and rupture in the same manner as eccrine sweat ducts and that
such blockages cause lumps, cysts, pain and tenderness in the
female breast.
Accordingly, the inventor believes these conditions are
influenced by exocrine function. Similarly, the inventor
believes that blockage of the ducts of the prostate gland in men
causes enlargement and pressure.
Furthermore, dry eye conjunctivitis and glaucoma can be caused
by the blockage of exocrine ducts; and blockage or malfunction
of exocrine glands of the mucosal surfaces of the
gastro-intestinal tract is a cause of many inflammatory diseases
including gastritis and colitis.
Moreover,
the inventor has found that such inflammation of the
gastrointestinal tract, particularly inflammation of the
intestines, might induce autoimmune responses which may cause
diseases such as,
Crohn's
disease, rheumatoid arthritis, osteoarthritis, systemic
lupus erythematosus, ankylosing spondylitis, multiple
sclerosis, motor neurone disease, polycystic ovarian
syndrome, mitral valve syndrome, diabetes type 1,
scleroderma, autoimmune thyroiditis, Graves disease and many
others.
The
blockage or malfunction of exocrine ducts of the lungs and
gastro-intestinal tract is believed to involve a loss of mucosal
surface innate immunity, thereby leaving the subject open to air
or fluid borne infection.
The
inventor therefore recommends that such conditions should be
cleared up in persons likely to be exposed to pathogens, such as
patients and staff in hospitals and before any medical
procedures or medication programmes, including vaccinations, are
commenced.
Also, especially in elderly persons who have extensive blockage
of exocrine glands within skin, a lack of protection by skin
anti-microbial peptide may lead to the easy entry of resident or
common pathogens through the skin, potentially leading to severe
infections.
It is well known that the disease of type 2 diabetes is
associated with hypertension and with serum hyperglycaemia which
is otherwise normally controlled by insulin hormone. It is the
contention of the inventor that insulin producing pancreatic
ducts function in the same manner as exocrine ducts.
The excess
output of insulin stimulated by hyperglycaemia is modified in
passage through ducts and the modified insulin is no longer
effective. This is "insulin resistance", known to be a cause of
type 2 diabetes.
Furthermore the inventor believes that excess output of the
hormones of the stomach, intestines, liver and pancreas results
in a partial loss of normal function of these hormones, leading
to a loss of control of appetite. Thus any weight loss programme
for obesity can be assisted by including treatment of hormone
producing exocrine ducts.
It is the object of the present invention to provide treatment
for Miliaria and other disorders partially caused by the
blockage or other malfunction of exocrine ducts.
According to a first aspect of the present invention there is
provided the use of sodium chloride formulated such that it
cannot cross epithelial barriers in the manufacture of a
medicament for the treatment of medical conditions at least
partially characterised by blockage or other malfunction of
exocrine glands. For example, the blocking or malfunctioning may
be of an exocrine duct.
In particular, the sodium chloride may be coated with an agent
that forms a liquid impermeable but gas permeable layer. In this
and other aspects of the present invention, the coated agent may
prevent passage through the coating of the substance or agent
(e.g. sodium chloride) encapsulated within it.
The coating
may be considered to be an encapsulation of the sodium chloride
(or other substances detailed below which are coated).
Thus
administration of the substance (the sodium chloride or other
agents as detailed below, coated with / encapsulated by the
agent) to a patient may result in no metabolic change to,
chemical change to, or diminution of, the sodium chloride (or
other substance as detailed below).
The coating
surrounding the sodium chloride (e.g. sodium chloride crystals
or granules) may be any liquid impermeable but gas permeable
barrier which prevents the sodium chloride from passing into
(e.g. when ingested) or onto the body. The agent may be ceramic
(e.g. a clay or an inorganic non-metallic material), a polymer
or a natural wax.
In the case of sodium chloride, it can be encapsulated to form a
sphere, for example of a diameter of 1-10 mm. For example, the
sphere may comprise sodium chloride crystals coated with bees
wax hardened with cornstarch and talc.
Sodium chloride or common salt (chemical formula NaCl) occurs
naturally in many parts of the world as the mineral, halite and
as mixed evaporates in salt lakes, by mass, sodium chloride is
60.663% elemental chlorine (Cl) and 39.337% sodium (Na).
The sodium chloride may be in crystal form. Sodium chloride
crystals are cubic in form and represent a preferred form of
sodium chloride for use according to the first aspect of the
invention.
It is more preferred that the sodium chloride is encapsulated by
a coating agent. Such an agent should encapsulate sodium
chloride crystals such that the coating is liquid impermeable
but gas permeable. The inventor has termed such encapsulated
crystals "Sensezero Therapeutic Inert Agent".
Encapsulated sodium chloride crystals represent an important
feature -of the present invention. Therefore, according to a
second aspect of the present invention there is provided
medically efficacious compound coated with an agent that forms a
liquid impermeable but gas permeable layer for use as a
medicament.
It will be
appreciated that the coating of the second aspect of the
invention may be applied to a number of medically useful
compounds. It is most preferred that the compound is sodium
chloride.
According to the various aspects of the present invention, the
coating may be any liquid impermeable but gas permeable barrier
which prevents the sodium chloride (or other substances detailed
below) from passing into (e.g. when ingested) or onto the body.
The agent may be ceramic (e.g. a clay or an inorganic
non-metallic material), a polymer or a natural wax. Thus sodium
chloride crystals or granules can be coated with beeswax
hardened with cornstarch and talc.
It should be noted that although sodium chloride is not normally
considered to be a medically efficacious substance, when used in
the various aspects of the present invention, it is medically
efficacious.
In a preferred embodiment of the second aspect of the present
invention, sodium chloride is encapsulated in a sphere with an
approximate diameter of 1 mm to 10 mm, preferably 3 mm to 8 mm,
but mostly preferred 6 mm. The sphere may comprise sodium
chloride crystals (which may be compressed) coated with beeswax
hardened with small amounts of cornstarch and talc.
The coating of beeswax hardened with small amounts of cornstarch
and talc is distinct from prior art tablet formulations and
suchlike which use wax to coat or encapsulate a medicament (for
example wax polishing of tablets). In such cases, the wax is
water permeable and is used to effect a slow or delayed release
of the medicament, or to enhance the appearance of the
medicament.
This is in
contrast to the present invention in which the coating (even in
the case of a wax-based coating) is water impermeable but gas
permeable. The hardening of the beeswax with cornstarch and talc
effects this required water impermeability.
It should be noted that normal dietary sodium chloride is not
effective according to the present invention because such salt
is able to be absorbed into the body by gastro-intestinal tract
epithelia upon ingestion. Salt used according to the present
invention is prepared such that this may not occur (e.g.
according to the second aspect of the invention).
The following medical conditions are amongst the disorders that
may be treated (prophylactically or when symptoms arise) by the
use of sodium chloride according to the present invention:
Miliaria crystallina; Miliaria rubra; Miliaria type 3;
Miliaria profunda; Chronic erythema; Flushing and blushing
with cyclical crops of pustules and papules; Tissue
hyperplasia; Disfiguring phyma; Rhinophyma; Rosacea;
Telangiectasia; Essential hypertension; Migraine;
Neurodegeneration; Cellulite; Asthma; Allergic rhinitis; Hay
fever; Atopic eczema; Lumps and cysts of the breasts;
Prostate gland enlargement; Dry eye conjunctivitis;
Glaucoma; Inflammation of the gastro-intestinal tract;
Gastritis; Colitis; Crohn's disease; Rheumatoid arthritis;
Osteoarthritis; Systemic lupus erythematosus; Ankylosing
spondylitis; Multiple sclerosis; Motor neurone disease;
Polycystic ovarian syndrome; Mitral valve syndrome; Diabetes
type 1; Scleroderma; Autoimmune thyroiditis; Graves disease;
Diabetes type 2; Hypertension associated with diabetes type
2; Acne vulgaris; and Obesity.
Herein, by
"treatment" is meant any treatment which is designed to cure,
alleviate, remove or lessen the symptoms of, or prevent or
reduce the possibility of contracting any disorder or
malfunction of the human or animal body.
The term
"treatment" also includes the treatment of e.g. skin conditions
such as cellulite (which may be considered to be cosmetic and
not a bodily disorder or malfunction) and the maintenance or
promoting of optimum health or cosmetic appearance of the human
or animal body.
Similarly,
reference herein to "medically efficacious" substances is to
substances which can be used to effect a treatment. Therefore a
medically efficacious substance includes substances which can be
used to effect the therapy or prophylaxis of a condition.
A preferred method for general treatment of the abovementioned
conditions involves the application of sodium chloride so that
it is in proximity to the blocked exocrine gland duct. It will
be appreciated that the precise way in which sodium chloride is
formulated and administered will depend on the individual
conditions to be treated.
It will also be appreciated that the amount of sodium chloride
that is required is determined by biological activity and
bioavailability which in turn depends on the mode of
administration, the physicochemical properties of any agent
employed to coat the salt and whether sodium chloride is being
used as a mono-therapy or in a combined therapy.
The
frequency of administration will also be influenced by a number
of factors and particularly the health status of the subject
being treated.
Optimal dosages to be administered may be determined by those
skilled in the art, and will vary with the particular condition
being treated, the strength of the preparation, the mode of
administration, and the advancement of the disease condition.
Additional factors depending on the particular subject being
treated will result in a need to adjust dosages, including
subject age, weight, gender, diet, and time of administration.
According
to the present invention, the treatment of different types of
Miliaria or other disorders described above may involve placing
sodium chloride crystals in proximity to skin.
This may be achieved by any method of adhering salt crystals to
skin but preferably avoiding direct contact between the salt and
the skin. Preferred methods include the use of adhesive tape and
Nelcro bandages. However, the most preferred way of placing
sodium chloride in proximity of the skin is by formulation of a
patch.
The patch
may be a patch of any type of sticking device provided it holds
sodium chloride crystals close to the skin. The inventor has
termed such patches "Sensezero Therapeutic Inert Patches".
Such patches represent an important feature of the present
invention and therefore in a third aspect of the present
invention there is provided a patch suitable for adherence to
skin containing sodium chloride adapted for use in the treatment
of medical conditions at least partially characterised by the
blockage or other malfunction of exocrine glands.
In a preferred embodiment of the third aspect of the present
invention, the patch may be any type of sticking plaster
suitable for adherence to skin, more preferably a water
resistant plaster and most preferably a hypoallergenic water
resistant plaster. The patch may be of any shape, however
preferably it is in the form of a figure eight.
According to a most preferred embodiment of the third aspect of
the present invention there are provided two spherical granules
of pure sodium chloride on- the patch and preferably, the sodium
granules are held at a distance of 1 mm to 1000 mm apart, but
most preferably the granules are held at 30 mm apart.
Preferably,
the granules may be of 1-10 mm diameter but more preferably
granules are of 2.5 mm diameter and have a coating. This coating
is preferably as described by the second aspect of the present
invention and is composed of water impermeable but gas permeable
material which prevents delivery of the sodium chloride across
the skin.
Another preferred method of placing sodium chloride crystals in
proximity to skin is the use of a device which temporarily and
at intervals places sodium chloride crystals against skin for a
therapeutic purpose. The inventor has termed this "Sensezero
Therapeutic Device".
Such a device represents an important feature of the present
invention and therefore in a fourth aspect of the present
invention there is provided a device consisting of a holder
adapted for holding a medically efficacious compound, an energy
source and an actuator driven by the energy source for
temporarily and at intervals placing the compound against the
skin of a subject.
The device
may be for treatment of conditions at least partially caused by
the blockage or other malfunction of exocrine glands.
According to the fourth aspect of the present invention, such a
device may be a miniature encased device made from any material,
preferably made from plastic. The device may be worn anywhere on
the body but more preferably on the abdomen or thorax of a
subject. Within the casing there may be source of energy such as
a low voltage battery in addition to an electronic timer.
The
actuator may be a spring return push-rod solenoid.
The device
may be adapted to hold sodium chloride. Preferably, pure sodium
chloride crystals are placed in the holder. The sodium chloride
may be coated with a coating according to the second aspect of
the present invention. Such coated sodium chloride is water
impermeable but gas permeable and thereby prevents delivery of
salt across the skin.
Daily doses may be given as a single administration.
Alternatively, the sodium chloride used may require
administration twice or many times during a day. As an example,
sodium chloride according to the invention may be administered
by wearing the device continuously for as many days as required
the device being activated for a few minutes in each hour.
Alternatively a patient may take a daily dose comprising one
patch which is replaced in a different position on the skin
after each 24 hours.
Conditions at least partially characterized by a blockage or
other malfunction of exocrine ducts may be treated with any one
of the approaches encompassed by the first, second, third and
fourth aspects of the present invention, For instance, a patch
according to the third aspect of the invention may be applied in
the abdominal region.
According to the present invention, the treatment of different
types of Miliaria or other disorders at least partly
characterized by blockage or other malfunction of exocrine ducts
may involve placing sodium chloride crystals in proximity to
skin. All conditions (skin and non-skin) may be treated in the
same way (e.g. with a patch according to the third aspect of the
invention on the skin, or alternatively use of the device
according to the fourth aspect of the invention).
Treatment
can be reinforced and enhanced by simultaneously using a coated
compound according to the second aspect of the invention which
can also be used as a mono-therapy.
Skin disease on the face, legs or arms may be treated by placing
a patch according to the third aspect of the invention or device
according to the fourth aspect of the invention on the abdomen
or thorax, on unaffected skin if possible. This is preferably
done with the additional use of a coated compound according to
the second aspect of the invention in most cases.
Non-skin
disease may be treated in the same way. Some therapists might
want to use a patch according to the third aspect of the
invention or a device according to the fourth aspect of the
invention or a coated compound according to the second aspect of
the invention as a mono-therapy. Following successful treatment
of a disease, one of the three might be used periodically as a
prophylactic.
However, it
is preferred that the treatment of any of the conditions is
enhanced synergistically by introducing into the body, orally or
otherwise, sodium chloride in the form defined by the second
aspect of the invention.
It will be appreciated that the invention (in all its aspects)
is particularly useful for treating human subjects. However the
subject may be any other mammal of veterinary interest.
Although the inventor does not wish to be bound by any
hypothesis, it is believed that sodium chloride can be used for
the treatment of medical conditions at least partially
characterized by blockage or other malfunction of exocrine ducts
for the following reasons .
The inventor has considered the behavior of modern humans in
advanced societies and has found that a change from an original
genetic habituation (i.e. the status of Man in a natural
habitat) to an adverse habituation (i.e. Man's unnatural or
modern habitat) can result in several health problems including
Miliaria disease and those mentioned above. It is believed that
such change may manifest in an altered physiology of exocrine
glands and particularly sweat glands.
The inventor believes that normal sweat glands output sweat
either at a basal level or at a higher level. This dual status
of sweat glands is believed to be the natural state and the
inventor has named this status as original genetic habituation
of sweat glands.
In this natural state all sweat glands continuously produce
sweat at the- basal level. In general, increasing exercise or
exposure to heat causes more and more sweat glands to become
involved at the higher level.
Regular
vigorous exercise or exposure to heat is essential for
maintaining the genetic habituation status of sweat ducts, as
they lead to abundant flow of sweat resulting in clear ducts due
to the physical force of fast flowing fluid. Persons who do not
exercise regularly, who encounter emotions like stress and who
live in artificial and temperate climates experience repeated
episodes of slightly greater sweat output than the basal level.
The
inventor believes that, as a consequence, re-absorption of
sodium by the duct becomes fixed at a slightly greater level
causing the duct to remain in a state of adverse habituation
even though the stimulus to sweat ceases. This acquired state
predispositions an affected person to disorders related to the
blockage of sweat glands since the salinity of the sweat sinks
below the levels required for efficient skin protection against
microbial pathogens by anti-microbial peptide.
This
enables microbes to enter ducts, which then become blocked by an
ensuing immune reaction.
During the initial stages of investigation of potential
treatments of the abovementioned medical conditions, the
inventor considered oceanic unicellular prokaryotes which
existed at the beginning of the evolutionary time. These, like
modern cells, actively transport sodium in and out to maintain a
lower sodium internal environment.
Since any
body of water containing dissolved salts is subject to changes
of concentration following natural flows and stratification, the
inventor concluded that, for survival, the earliest prokaryotes
must have been able to detect internal and external elements of
dissolved molecules, and act on the information. The inventor
termed this detection ability "sensezero".
The inventor then pictured the human body enveloped in fluid,
with the skin and the lungs in contact with fluid in the form of
a gas, i.e. air, and the gastro intestinal tract in contact with
liquid. The inventor concluded that, if the body senses the
presence of surplus sodium in both the gas and the liquid
environment, exocrine glands in adverse habituation are free to
reset to genetic habituation.
Thus, according to the present invention, the basis for the
treatment and prophylaxis of Miliaria and diseases mentioned
above is to provide in the air and in the liquid environment of
the body an amount of sodium, which appears to indicate a
surplus to allow resetting to genetic habituation.
Furthermore, to prevent desensitization of the body to sodium
chloride and the wearing off of the therapeutic effect, the
inventor found that treatment and prophylaxis have to be
arranged so that the body can sense new additional sodium salt
without absorbing it. Accordingly, salt used according to the
present invention should be made not to cross epithelial
barriers.
Also it is now known that in multi-cellular animals complex cell
signaling systems are used for motility, for survival and
apoptosis, for example, and that these signals affect or control
the maintenance of health and the progress of disease.
The inventor has also noted that many drugs which are formulated
to pass into body circulation are not in fact metabolized in the
body, but appear to achieve their therapeutic effect by their
presence, and are then excreted unchanged.
The inventor therefore concluded that many drugs could have a
therapeutic effect by being present in the environment of the
body, but without entering circulation. The environment of the
body he considered to be fluid. In the case of the skin and
lungs the fluid is a gas i.e. air, and in the case of the
gastrointestinal tract it is liquid.
The inventor does not wish to be bound by the hypothesis, but
believes that the presence of a drug in the body environment but
not in body circulation influences cell signaling and
sense-zero, and can therefore have a desired therapeutic effect
without the drug being in circulation.
Thus as well as being applicable to sodium chloride, the above
findings and each of the above aspects of the invention are also
applicable to medicaments, patches and devices incorporating
substances other than sodium chloride. In particular, the sodium
chloride in the above described aspects of the present invention
may be replaced by capsaicin, metformin, salicylic acid, or a
derivative of salicylic acid thereof.
Additionally, the sodium chloride may be replaced by a substance
endogenous to the body (e.g. the human or animal body as
appropriate, depending upon the intended recipient of any
medication or treatment), a food substance, or a drug.
Many thousands of compounds are currently manufactured for use
for therapeutic purposes. Naturally occurring substances, for
example plant material, are also prepared for use for
therapeutic purposes. For the present purpose, these compounds
and substances are teπned "drugs".
Drugs are almost always compounds foreign to the body. As such,
they, unlike endogenous substances, are not continually being
formed and eliminated. Drug absorption, bioavailability,
distribution, and elimination are therefore determinants of
onset, duration, and intensity of drug effect.
Drug absorption in mammals is determined by their
physicochemical properties, their formulations and routes of
administration. The actual dosage forms (e.g. tablets, capsules,
solutions) consisting of the drag and suitable excipients are
formulated to be administered by various routes including oral,
buccal, sublingual, rectal, parenteral, dermal and inhalational.
A prerequisite to absorption is drug dissolution. For example,
solid drug products like tablets disintegrate and disaggregate
quickly or slowly, but absorption can only occur after drugs
enter solution.
Drugs are designed to enter systemic circulation to have the
desired effect. Thus those skilled in the art of drug
preparation are concerned to achieve effective transport across
biological barriers, to control transit times, dissolution and
absorption, and to maximize bioavailability in circulation and
at the place of the therapeutic target.
Some drugs
cross the cell membrane to enter the cell itself. Drugs in
circulation may also metabolize in the body in a wide range of
chemical reactions including oxidation, reduction, hydrolysis,
hydration, conjugation, condensation and isomerisation and these
reactions have to be carefully predicted.
Unwanted
metabolites may be difficult to eliminate from the body. There
may also be harmful interactions with other drugs or with
endogenous substances.
Drugs in circulation can accumulate over time in tissues or body
compartments and thus cause undesirable effects. Drugs in
circulation may penetrate areas where they would be harmful,
such as across the blood-brain barrier or the placenta.
Therefore great skill is needed in the manufacture and testing
of drugs, and the costs of formulating current drugs are very
high, often hundreds of millions of pounds. Nevertheless, nearly
all drugs in circulation have undesirable side effects, and may
harm the user. Despite very strict regulation by government
agencies, in the USA over 100,000 deaths per annum are
attributed to adverse reactions to approved drugs.
Thus according to a fifth aspect of the present invention there
is provided a preparation comprising a medically efficacious
substance coated or otherwise enclosed by an agent that forms a
liquid impermeable but gas permeable layer for use as a
medicament.
The inventor has termed this "ActivSignal" (TM) class of drugs.
The agent may be formulated such that the substance cannot cross
epithelial barriers in mammals. The agent may be formulated such
that the substance cannot cross membrane barriers in mammals.
The agent may be formulated such that the substance cannot cross
into cells of mammals.
The
medically efficacious substance may be selected from the group
consisting of: sodium chloride, capsaicin, metformin, salicylic
acid, and a derivative of salicylic acid. It may be selected
from the group consisting of: a substance endogenous to the
body, a food substance, and a drug.
The preparation may be formulated in a variety of ways,
including for oral, buccal, nasal, sublingual, rectal,
parenteral, topical, dermal or inhalational administration and
use, the formulations including nanoparticle and microparticle
forms.
For example, the preparation may be in the form of a pill, a
tablet, a lozenge, a bolus, a capsule, a caplet, a granule, a
nanoparticle, or a microparticle. It may be in granular,
nanoparticle or microparticle form as a suspension, a cream, or
a paste.
The fifth aspect of the present invention provides for drugs to
have a therapeutic effect without the undesirable side effects
of drugs put into circulation, such as accumulation, toxicity or
possible patient overdose.
The
invention gives the drug a consistent therapeutic effect as the
"dose" is a constant and there is no metabolic change or
metabolic by product. The invention may improve the shelf life
of drugs by excluding moisture. In the present invention some
endogenous body substances may be used for a therapeutic effect,
as such substances are formulated not to pass into circulation
where they would have no effect.
A preparation according to any of claims 26-29, prepared for use
with a patch for holding said preparation near to or against the
skin of a patient.
The preparation may be for implantation into the body of a
patient.
The agent may be a ceramic, a polymer, a natural wax, or beeswax
hardened with cornstarch and talc. The substance may be sodium
chloride, capsaicin, metformin, salicylic acid, or a derivative
of salicylic acid.
The substance may be a substance endogenous to the body, a food
substance, a plant material, or a drug.
Such preparations may be combined with a preparation of at least
one ingredient designed for delivery into solution, particularly
with a preparation of at least one ingredient designed for
delivery into solution for a therapeutic purpose.
In one embodiment the active ingredient is formulated into oral
administration tablets together with the excipients natural
beeswax, cornstarch and talc. This method of manufacture is well
known to those skilled in the art. Wax matrices are widely
employed for drug delivery throughout the pharmaceutical
industry because of the low production cost and ease of
manufacture.
In
conventional drug delivery tablet manufacture the proportion of
excipients by weight in the tablet is in the range of
approximately 1% to 5%, and designed to facilitate immediate or
delayed release of the active ingredient into solution.
In this
most preferred embodiment the excipients, mostly wax plus a
small amount of cornstarch and talc, are a proportion of the
tablet mixture between 20% and 45% by weight. This novel method
of manufacture is designed not to release any of the active
ingredient into liquid solution, but the wax matrix is
inherently gas permeable.
In a more preferred embodiment, the active ingredient of the
tablet is -enclosed by a polymer and formulated to be liquid
impermeable, but gas permeable. In preferred embodiment, the
active ingredient may be enclosed by a ceramic and formulated to
be liquid impermeable, but gas permeable.
In a fourth
embodiment the active ingredient may be enclosed within a metal
tablet or capsule, such as perforated stainless steel, allowing
passage of a gas but not liquid.
Other
embodiments include a pill, lozenge, bolus, capsule, caplet,
granule or any suitable type, size or shape of manufacture,
administered by any route, wherein the active ingredient is
protected from contact with liquid but which is potentially
contactable by a gas.
It is desirable to test the integrity of the liquid
impermeability characteristic of a formulation, and that its
physical integrity is preserved during use. A suitable in vitro
test for a tablet, for example, is agitation in water adjusted
to a pH of 3 with hydrochloric acid for three hours, followed by
agitation in water adjusted to a pH of 7 with sodium bicarbonate
for a further twenty four hours. The medication should not be
changed to any significant degree by this test.
In vivo, after oral administration, a tablet may tested by being
recovered from faeces, and should be found to be unchanged, or
only changed to an insignificant degree, after passage through
the body.
Drugs formulated according to the present invention may be
formulated in nanoparticle or microparticle size. US 4622244
discloses the microencapsulation of an active by a suitable
polymer to produce microcapsules of less than 300 microns in
size i.e. suitable for injection in a suspension medium by means
of small needles customarily employed in medical practice and
thereby achieving controlled or sustained release of the active
into body circulation.
This method
of manufacture is well known to those skilled in the art, and in
the instance of the present invention the manufacture is
achieved by encapsulation by a wax or polymer or other suitable
barrier which is liquid impermeable but gas permeable.
Any embodiment may be made up for monotherapy or combined for
multiple therapy as convenient. Any embodiment may include more
than one active ingredient as convenient. Any embodiment may be
combined with a preparation of any other active ingredient
prepared for immediate or delayed or selective delivery into
solution, as convenient.
Since the
therapeutic effect of the invention depends on the presence of
the drag in the environment of the body, arrangements can be
made to prevent desensitization over time or the wearing off of
the therapeutic effect. In the case of oral administration,
desensitization is avoided by the constant movement of the
medicament through the gastrointestinal tract.
In the case
of administration by other routes it may be necessary for the
present invented medicament to be provided and withdrawn at
intervals, for example provided for three minutes in each thirty
minutes, to avoid desensitization. For example, a tablet of an
ActivSignal class drug may be placed on the skin and withdrawn
at intervals by use of a device including an electrical or other
energy driven actuator held near to the skin of a subject.
The same
method is used where it is desired to surgically or otherwise
implant an ActivSignal class drug in the body of a subject.
Alternatively, very small quantities of the preparations may be
used at longer intervals.
For example
a dermal patch may be provided with two pellets of the drug (the
medically efficacious substance) formulated according to the
present invention and of 2-3 mm diameter and placed about 30 mm
apart with the patch being moved to a different location on the
skin after e.g. every twelve hours.
Also provided according to a sixth aspect of the present
invention is a method of manufacture of a medicament comprising
coating or otherwise enclosing a medically efficacious substance
in an agent that forms a liquid impermeable but gas permeable
layer.
The various features of the other aspects of the present
invention as discussed above apply equally to the sixth aspect
of the invention.
It will be appreciated that the amount of the drag used
according to the present invention and the physiochemical
properties of any agent employed to coat or otherwise enclose
the drag will be influenced by the route of administration as
well as a number of other factors including the health status of
the subject being treated.
The
invention will be further apparent from the following
description, with reference to the several figures of the
accompanying drawings, which show, by way of example only, forms
of the present invention.
Of the
Figures: Figure 1 illustrates a patch according to the third
aspect of the invention. Figure 2 illustrates a device according
to the fourth aspect of the invention; and Figure 3 illustrates
a section view of a device according to the fourth aspect of the
invention.
In Figure 1 : "A" illustrates a patch according to the third
aspect of the invention showing the obverse adhesive coated side
comprised of hypoallergenic water resistant plaster with two 2.5
mm diameter spherical granules of coated sodium chloride
approximately 30 mm apart and fixed to the adhesive of the
plaster; "B" illustrates the reverse side of the same
hypoallergenic water resistant plaster; and "C" illustrates a
side view of the hypoallergenic water resistant plaster showing
two 2.5 mm spherical granules of coated sodium chloride
approximately 30 mm apart and fixed to the adhesive of the
plaster.
Figures 2 and 3 illustrate a device according to the fourth
aspect of the invention. The scale is approximately 3 to 1.
These figures illustrate a block of coated sodium chloride
approx 12 mm x 13 mm x 4 mm (1); a spring return push rod
solenoid (2); a low voltage battery (3); a electronic timer (4);
handles to take belt or strapping (5); and a plastic casing for
the device to be placed against the skin (6). EXAMPLES
EXAMPLE ONE BACKGROUND
Cayenne
pepper, a common food ingredient, is extracted from the chilli
pepper (Capsicum annum) seed pod.
The active
ingredient of the pepper is the alkaloid capsaicin. Creams or
lotions containing 0.025-0.075 % capsaicin are on sale and have
a long history of use in deπnatology for the treatment of
itching and pain.
When
applied to the skin, capsaicin causes a burning sensation
associated with depletion of neuropeptides from nociceptor nerve
endings. Successful suppression of itch by topical
administration has been reported for a number of praritic
dermatoses (Reimann S et al., "Topical administration of
capsaicin in dermatology for treatment of itching and pain",
Hautarzt. 2000 Mar;51(3): 164-72; PMID: 10789077).
Atopic eczema (dermatitis) is a highly pruritic skin disease
with patches of inflammation, weeping, blistering and bleeding
if scratched.
Many
sufferers have disturbed sleep due to the constant itching. The
open nature of the inflammation means that topical capsaicin
with its burning sensation cannot be used to relieve eczema
pruritus.
Indeed
topical capsaicin can induce dermatitis.
METHOD
Cayenne pepper was coated with a formulation of beeswax hardened
with a small quantity of cornstarch and talc and compressed to
form spherical pills of about 7 mm diameter. According to the
present invention these were made to be gas permeable but liquid
impermeable ActivSignal class drags.
Samples of
the pills were agitated in vitro in acidic water for 4 hours,
and alkaline water for 24 hours.
The pills
remained intact and no cayenne pepper was found in the water.
Six adults with atopic eczema (dermatitis) who had complained of
pruritus and who were solely using topical preparations for
relief were recruited.
After
informed consent was obtained each was asked to take one of the
cayenne ActivSignal class pills at 3 pm and then not use their
topical medications until after 8 am the following morning. Each
was asked to continue the treatment in the same manner for seven
days. Each was asked each following day by telephone to rate
their relief from overnight pruritus.
Four
subjects reported 100% relief on each of the seven nights as a
result of the ActivSignal class drug treatment. One subject
reported 90% relief and one reported 50% relief as a result of
the ActivSignal class drug treatment. No adverse side effects
were reported. Two subjects reported a feeling of warmth and
slight sweating 2-3 hours after the first occasion of taking the
pill.
It is concluded that a gas permeable but liquid impermeable
preparation of cayenne pepper according to the present invention
is effective for the treatment of eczema pruritus, with no
adverse side effects reported.
EXAMPLE TWO BACKGROUND
Metformin,
a biguanide, has been available in the USA for the treatment of
type 2 diabetes mellitus for nearly 8 years and in Europe for
over 20 years.
Over this
period of time, it has become the most widely prescribed oral
anti-hyperglycaemic agent. Its mechanism of action involves the
suppression of endogenous glucose production, primarily by the
liver. Whether the drag actually has an insulin sensitising
effect in peripheral tissues, such as muscle and fat, remains
somewhat controversial.
Nonetheless, because insulin levels decline with metformin use,
it has been termed an 'insulin sensitiser'. Metformin has also
been shown to have several beneficial effects on cardiovascular
risk factors and it is the only oral anti-hyperglycaemic agent
thus far associated with decreased macrovascular outcomes in
patients with diabetes.
Cardiovascular disease, impaired glucose tolerance and the
polycystic ovary syndrome are now recognised as complications of
the insulin resistance syndrome, and there is growing interest
in the use of metformin for these extraordinarily common
metabolic disorders. While diet and exercise remain the
cornerstone of therapy for insulin resistance, pharmacological
intervention by use of metformin is now a well used alternative.
Metformin, however, is thought to sometimes accumulate in the
body and thus increase the risk of lactic acidosis, a
potentially fatal condition.
Metformin
therefore is considered to be contraindicated in many chronic
hypoxemic conditions that may be associated with lactic
acidosis, such as cardiovascular, renal, hepatic and pulmonary
disease, and advancing age.
METHOD
Metformin
250 mg was coated with a formulation of beeswax hardened with a
small quantity of cornstarch and talc and compressed to form
spherical pills of about 7 mm diameter.
According
to the present invention these were made to be gas permeable but
liquid impermeable ActivSignal class drugs. Samples of the pills
were agitated in vitro in acidic water for 4 hours, and alkaline
water for 24 hours. The sample pills remained intact. On
dissection no liquid was found to have entered the sample pills.
Five adults suffering from diabetes type 2 controlled by
metformin alone were recruited. The group had been diagnosed
with fasting plasma glucose (FPG) in the-range 10 to 15 mmol/1
before commencing the metformin treatment.
Current
dosages ranged from 500 mg metformin twice daily to 850 mg
metformin three times daily. The five adults were controlling
their FPG to below 8 mmol/L and were taking weekly measurements
with results in the range 5 to 8 mmol/L. Three of the five
recalled having a metallic taste in the mouth occasionally and
all five reported occasional abdominal discomfort as
side-effects of taking metformin.
With informed consent, the five adults agreed to substitute the
ActivSignal metformin for the regular metformin at the rate of
one ActivSignal metformin pill for each regular metformin tablet
they were currently taking, for a period of four weeks.
During the four week trial all of the five adults reported that
they were controlling their FPG to below 8 mmol/L whilst taking
the ActivSignal metformin. No side effects were reported.
It is concluded that ActivSignal metformin according to the
present invention has the equivalent therapeutic effect to
regular metformin for persons suffering from moderate diabetes
type 2, but that ActivSignal metformin has reduced or no side
effects.
In
addition, since ActivSignal metformin is not released into the
body there can be no accumulation, so that ActivSignal metformin
is likely to be able to be used where currently contraindicated
for the therapy of persons with chronic conditions that may be
associated with lactic acidosis.
EXAMPLE THREE BACKGROUND
Aspirin is the acetyl derivative of salicylic acid that is used
to lower fever, relieve pain, reduce inflammation, and thin the
blood.
Common
conditions treated with aspirin include headache, muscle and
joint pain, and the inflammation caused by rheumatic fever and
arthritis. Aspirin is believed to act against fever, pain, and
inflammation by interfering with the synthesis of specific
prostaglandins in the body. Because of its ability to inhibit
the formation of blood clots, aspirin is also used in low doses
to prevent heart attack and stroke and to control unstable
angina.
The drag's
usefulness in preventing certain cancers, the dangerous high
blood pressure that sometimes occurs during pregnancy (toxemia),
and migraine headaches is also under investigation.
Normal dosage may cause nausea, vomiting, diarrhoea, or
gastrointestinal bleeding. Large doses cause acid-base imbalance
and respiratory disturbances and can be fatal, especially in
children.
Aspirin
also has been linked to the development of Reyes' syndrome (a
combination of acute encephalopathy and fatty infiltration of
internal organs) in children who have taken it for viral
infections. Acetaminophen (paracetamol) which does not cause
gastric irritation, lowers fever and relieves pain but does not
reduce inflammation, is often substituted for aspirin. Ibuprofen
may be used instead of aspirin for up to ten days without
consultation with a physician.
Ibuprofen
may have similar side effects to aspirin although this is less
common.
Salicylic acid or 2-hydroxybenzoic acid, C6H4(OH)CO2H,
is colorless, crystalline organic carboxylic acid used as an
oral analgesic up to the end of the nineteenth century, until
the invention of the less irritating acetyl derivative, aspirin.
Other
derivatives of salicylic acid are used as an active ingredient
of many topical preparations including sun creams, toothpaste,
antiseptics and food.
Aspirin is
the most widely used medication in the world with over 80
billion doses sold annually in the USA alone, and aspirin is an
active ingredient in over fifty over-the-counter medications.
METHOD
Pharmaceutical grade aspirin was hydrolyzed to salicylic acid
and then coated with beeswax hardened with cornstarch and talc
and compressed to form pills of about 6 mm diameter.
According
to the present invention these were made to be gas permeable but
liquid impermeable ActivSignal class drags. Samples of the pills
were agitated in vitro in acidic water for 4 hours, and alkaline
water for 24 hours. The sample pills remained intact. On
dissection no liquid was found to have entered the sample pills.
Twelve adults were recruited who were taking aspirin or
acetaminophen (paracetamol) or ibuprofen ad lib for the relief
of mild to moderate arthritic pain, at up to the maximum
recommended dose per day, namely 12 x aspirin 300 mg, or 8 x
acetaminophen 500 mg or 6 x 200 mg ibuprofen. Some of the group
were taking combined aspirin and acetaminophen up to the
combined recommended daily dosage.
With informed consent members of the group agreed to substitute
their regular analgesic with ActivSignal salicylic acid pills
for three weeks.
They were
advised to start, when required, with one ActivSignal salicylic
acid pill per day or two (one morning, one evening) if required.
They were advised, if necessary, they could take (one at a time)
up to six pills per day with a minimum two hour interval. At the
end of the trial six persons reported that the ActivSignal
salicylic acid pills were more effective at relieving pain than
their regular analgesic.
A further
five persons reported that the ActivSignal salicylic acid pills
gave about the same level of pain relief as their regular
analgesic.
These
eleven persons had taken either one, two or three ActivSignal
salicylic acid pills on most days. None had found the need to
take more than three of the pills on any day. All reported that
the pain relief seemed to be longer lasting with the ActivSignal
salicylic acid pills than with their regular analgesic.
No side
effects were reported. One person found the ActivSignal
salicylic acid pills less effective than the ibuprofen she was
normally taking, and dropped out of the trial.
It is concluded that ActivSignal salicylic acid according to the
present invention is an effective and long lasting analgesic
with no reported side effects.
EXAMPLE FOUR
BACKGROUND
Essential hypertension is one of the major health problems of
the developed world, affecting over 20% of the adult population.
Essential
hypertension is defined as persistent high pressure of unknown
cause. Untreated essential hypertension can lead to heart attack
(myocardial infarction), congestive heart failure, other heart
damage, arteriosclerosis, kidney damage, stroke and loss of
vision.
The inventor has found that the main cause of essential
hypertension is the prevalence of asymptomatic miliaria
profunda in the population of advanced societies, this
disease causing inflammation which raises pressure in skin
capillaries.
The
miliaria is a result of over conservation of sodium by sweat
gland ducts. It follows that by using ActiveSignal class drugs
containing sodium the over conservation can be reversed, and the
miliaria and thus the essential hypertension is prevented.
The classification of blood pressure in adults by the World
Health Organisation and the International Society of
Hypertension (revised 1999) is as listed in Table 1.
Table 1

All values are mmHg. Measurements are taken with subjects in the
sitting position.
Sodium
chloride or common salt occurs naturally in many parts of the
world. By mass, sodium chloride is 60.663% elemental chlorine
and 39.337% sodium. Sodium chloride crystals are cubic in form
and are readily available as a pure chemical.
Sodium chloride is an endogenous substance of the mammalian
body, and essential to the maintenance of life, but ordinary
intake of the chemical has never been found to have a
therapeutic effect.
Indeed
restriction of sodium chloride is recommended for persons with
essential hypertension.
METHOD
Pure sodium chloride was coated with beeswax hardened with
cornstarch and talc and compressed to form pills of about 6mm
diameter ActivSignal class drug and granules of about 2 mm
diameter ActivSignal class drag.
Two each of
the granules were fixed to an adhesive hypoallergenic patch at a
distance of about 30 mm.
Following informed consent nine persons with measured mild,
moderate or severe hypertension were asked to participate in a
trial. Before commencement of the trial, the blood pressure of
each person was measured after the subject had been at rest
seated for fifteen minutes.
After a
further ten minutes the blood pressure was measured again and
the average of the two systolic readings and the average of the
two diastolic readings was noted. Measurements were taken using
the Omron 705IT Blood Pressure monitor, a clinically validated
machine.
Small,
medium and large cuffs were available and selected according to
the manufacturers instructions.
The subjects were then asked to fix the patch with the two
ActivSignal class drug granules anywhere on the front of their
abdomen. Further, a new patch was fixed in a different position
after each twenty four hours for a further three days and the
old patch discarded. The subjects were also given one of the
pill ActivSignal class drugs taken orally with about 200 ml
water on the first, and third days of the trial.
Blood pressures were taken in the same manner after 1, 2, 4 and
6 days of the trial.
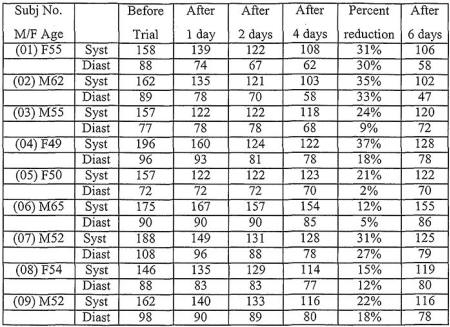
At the start of the trial four of the persons had mild
hypertension, three had moderate hypertension and two had severe
hypertension. After four days, measurements of blood pressure as
listed in Table 2 show that following treatment the systolic
blood pressure had been reduced by 27% and the diastolic
pressure by 18%.
After four days six subjects were now within the "optimum"
classification of blood pressure, and two were now within the
"normal" classification. One of the subjects originally having
severe hypertension was now classified as having mild
hypertension.
After six
days, as shown in Table 2, the benefits of the treatment
persisted after the treatment had been discontinued.
These data illustrate that the use of sodium chloride as an
ActivSignal class drag according to the present invention may
easily and rapidly reduce blood pressure to within normal limits
so that the subjects can no longer be considered hypertensive.
The treatment has the effect of restoring skin blood capillaries
to their natural free flowing function.
The invention has the effect of resetting blood pressure to what
is considered normal.
Essential hypertension is known to be only a slowly progressive
disease and it can therefore be anticipated that following
treatment the subjects are unlikely to become hypertensive again
for many months or some years, when the treatment can be
repeated.
Compared with treatments with current pharmaceutical products
which may need to be taken for a lifetime, which have unpleasant
side effects, and which do not treat the underlying disease, the
present invention is a much swifter, more effective and less
costly treatment of essential hypertension with no known side
effects.
Results are shown in Table 2.
Table 2