|
Western Washington University last updated 9/10/2004
from
AllesBiology-WesternWashingtonUniversity
Website
Introduction
As Neil de Grasse Tyson, an astrophysicist and the director of New York City’s Hayden Planetarium, has put it:
Allan Sandage on Stellar Evolution
For a while the number of protons and
neutrons was almost the same, until the temperature dropped enough
to make its slight mass difference favor the protons. Isolated
neutrons are not stable, so the ones that survived are the ones that
could bond with protons to form deuterium, helium, and lithium.
While the temperature was dropping, the
universe was also expanding, and the chances of collision were
getting smaller. Also very important is the fact that there is no
stable nucleus with 8 nucleons. So there was a bottleneck in the
nucleosynthesis that stopped the process there. In stars, this
bottleneck is passed by triple collisions of 4He nuclei (the
triple-alpha process), but in the expanding early universe, by the
time there was enough 4He the density of the universe had dropped
too much to make triple collisions possible.
Deuterium is easily destroyed by stars, and
there is no known natural process other than the Big Bang which
would produce significant amounts of deuterium.
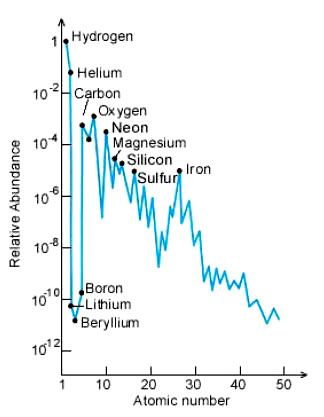
The observed abundance of baryonic matter in our universe shows hydrogen makes up ~75% and helium ~25% of ordinary matter. All the other elements are a small fraction of the total (~1%) and represent the material that has been subjected to high enough temperatures and densities in stars to burn helium and make the heavier elements. Observation therefore closely matches the theoretical predictions of the standard Big Bang model.
Note the chart uses a log scale in order
to show the rarer, heavier elements on the vertical axis.
Massive stars can synthesize elements heavier than oxygen (O); these stars eventually explode as supernovae. Elements heavier than iron are made in such explosions. The chart on the preceding page has a logarithmic scale, in which abundance increases by a factor of 10 for each unit of height.
Elements heavier than cadmium (Cd) are too rare to be displayed.
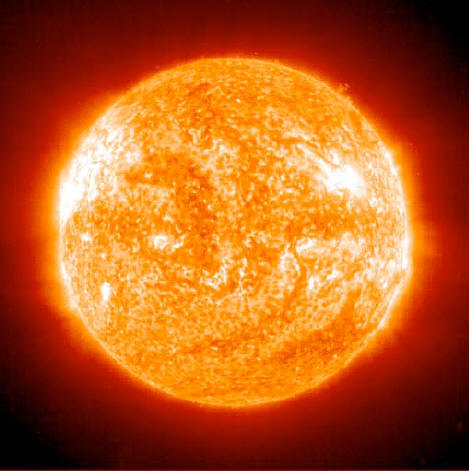
Stars, such as our Sun (above), are the only place in our universe
where the elements heavier than hydrogen and helium are produced.
Stars with a mass similar to our Sun can produce heavier elements up
to oxygen.
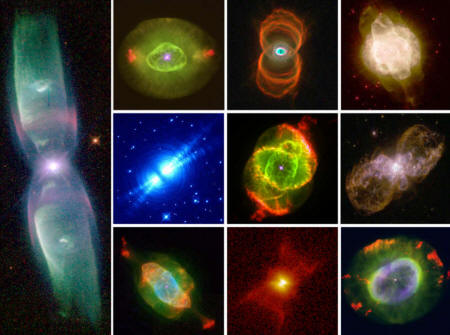
Planetary nebulae
are formed when a red giant star ejects its outer layers as clouds
of luminescent gas, revealing the dense, hot, and tiny white dwarf
star at its core. The other 5% of stars—that is, those born with
masses more than eight times larger than our Sun—end their lives as
supernovae.
Rather, they are the gaseous and dusty
material expelled by a geriatric star just before death. A far
better name for these objects would be "ejection nebulae". Think of
ejection nebulae as a cloud of smoke which escapes from a burning
log as it collapses and crumbles into embers.
The result is carbon nuclei and even
more heat. The "second wind" of heat release will be furious,
increasing the light emitted from the future Sun's surface by a
thousand fold. Meanwhile, the same heat will cause the outer layers
of the present Sun to expand and form a huge "red giant".
Eventually the outer 40% of the Sun's
mass will be spasmically "coughed" into space, floating outward
through the solar system and beyond in a concentric set of spherical
bubbles. Seen from far away, these may eventually blend together
into a gigantic stellar "halo". As the outer layers are flung
outward increasingly deeper and deeper layers of the Sun become
exposed as its outermost surface, like peeling an onion.
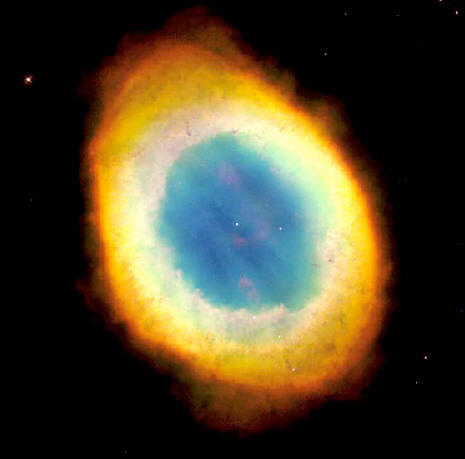
The Hubble Space Telescope captured the sharpest view yet of the Ring Nebula, the most famous of all planetary nebulae. In this image, the telescope has looked down a tunnel of gas cast off by a dying star thousands of years ago.
This photo reveals elongated dark clumps of material embedded in the gas at the edge of the nebula, and the dying central star floating in a blue haze of hot gas.
The nebula is approximately one
light-year in diameter, and is located some 2,000 light-years from
Earth. The colors are approximately true colors, and represent three
different chemical elements: helium (blue), oxygen (green), and
nitrogen (red).
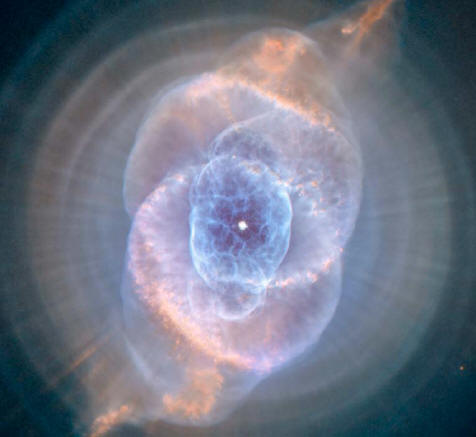
A classic planetary nebula, the Cat's Eye nebula (NGC 6543) seen above shows the final, brief yet spectacular phase in the life of a sun-like star.
This nebula's dying central star produced the simple, outer pattern of dusty concentric shells by shrugging off outer layers in a series of regular convulsions.
But the formation of the beautiful, more complex inner structures, shown in this close-up view, is not well understood. Seen so clearly in this sharp Hubble Space Telescope image, the nebula is over half a light-year across and lies three thousand light-years from Earth.
By observing the Cat's Eye nebula, astronomers may well be seeing
the fate of our sun, destined to enter its own planetary nebula
phase of evolution 5 billion years from now.
A montage of images of planetary nebulae made with the Hubble Space Telescope. These illustrate the various ways in which dying stars eject their outer layers as highly structured nebulae. Credits: Bruce Balick, Howard Bond, R. Sahai, their collaborators, and NASA.
The heaviest elements are produced in supernova explosions of
massive stars that are at least eight times the size of our Sun. The
Crab Nebula, pictured above, was produced by a supernova explosion
witnessed by Chinese astronomers in 1054 A.D.. Now approximately 10
light years in diameter, it is still expanding at about 1,100 miles
per second.
The mass accretion process most likely involves a merger of two discrete white dwarfs, which fuse briefly into a single unstable object, leaving behind a cooling, expanding debris remnant after the explosion. This process creates and disperses most of the chemical elements with atomic masses near and above that of iron.
The use of type Ia supernovae as probes
in studying cosmology is now well established.
Type Ib/c supernovae are associated with parent stars of more than about 30 solar masses and are probably closely related to, or may even be, the ultra luminous gamma-ray bursters, whereas stars with masses below this limit but above nine solar masses become various subtypes of type II supernovae.
Supernova 1994D, visible as the bright spot on the lower left, occurred long ago in the outskirts of disk galaxy NGC 4526. Supernova 1994D is not of interest for how different it was, but rather for how similar it is to other supernovae.
The light it emitted during the weeks
after its explosion identified it as a type Ia supernova, which are
of great interest to astronomers.
The more massive of the two stars will evolve faster and when it becomes a red giant it may be so big that gravity draws its outer atmosphere across to the companion star.
The transfer of material can lead to all kinds of interesting and exciting effects, depending on the properties of the two stars.
Stars that have lost their atmospheres to their companions are identical to the white dwarves in the center of planetary nebulae.
The less massive companion star, assisted by the extra mass it has gained, eventually becomes a red giant and starts to transfer material back onto its white dwarf companion. This can have the effect of increasing its mass beyond a critical limit of 1.4 times the mass of the Sun, known as the Chandrasekhar limit.
When this happens the carbon-oxygen core can suddenly explode, converting half the mass by nuclear fusion into elements like chromium, manganese, iron, cobalt and nickel. This is called a type Ia supernova. Because they are very bright and we think they always explode releasing about the same amount of energy, they are used as standard brightness light sources.
The recent discovery that the expansion
of the universe is accelerating, was made by observing type Ia
supernovae in galaxies 5,000 million light years away.
To support the weight of the star's
massive outer layers, the temperature and pressure in its core have
to be high. A star of 20 solar masses is more than 20,000 times as
luminous as the sun. Rushing through its hydrogen-fusion phase 1,000
times faster, it swells up to become a red giant in just 10 million
years instead of the Sun's 10 billion.
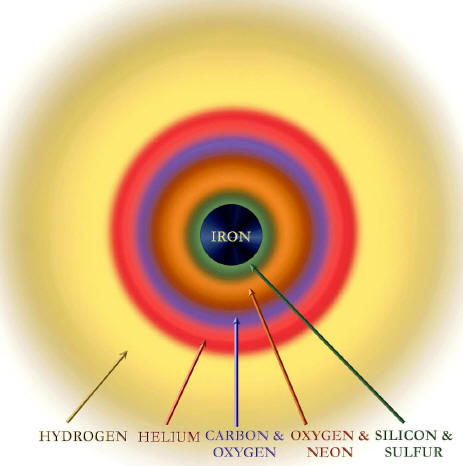
Silicon burns to make iron. Intermediate stages of fusion and decay make many different elements, all the way up to iron.
The iron nucleus occupies a special place in nuclear physics and, by extension, in the composition of the universe. Iron is the most tightly bound nucleus. Lighter nuclei, when fusing together, release energy.
To make a nucleus heavier than iron, however, requires an expenditure of energy. This fact, established in terrestrial laboratories, is instrumental in the violent death of stars. Once a star has built an iron core, there is no way it can generate energy by fusion. The star, radiating energy at a prodigious rate, becomes like a teenager with a credit card.
Using resources much faster than can be replenished, it is perched on the edge of disaster.
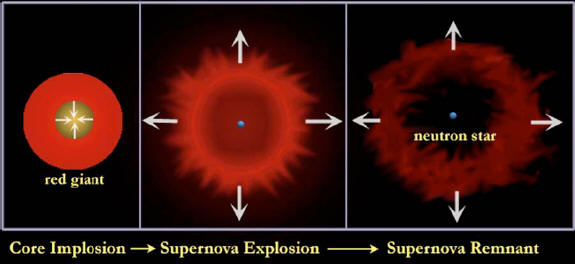
For massive stars disaster takes the form of a supernova explosion. The core collapses inward in just one second to become a neutron star or black hole. The material in the core is as dense as that within a nucleus. The core can be compressed no further. When even more material falls into this hard core, it rebounds like a train hitting a wall.
A wave of intense pressure traveling faster than sound —a sonic boom— thunders across the extent of the star. When the shock wave reaches the surface, the star suddenly brightens and explodes. For a few weeks, the surface shines as brightly as a billion suns while the emitting surface expands at several thousand kilometers per second.
The abrupt energy release is comparable to the total energy output of our Sun over its entire lifetime.
Such type II supernova explosions play a special role in the chemical enrichment of the universe.
First, unlike stars of low mass
that lock up their products in white dwarfs, exploding stars eject
their outer layers, which are unburned. They belch out the helium
that was formed from hydrogen burning and launch the carbon, oxygen,
sulfur and silicon that have accumulated from further burning into
the gas in their neighborhood.
For every 100 billion hydrogen atoms,
there is one uranium atom—each made at special expense in an
uncommon setting.
This theoretical picture of the creation of heavy elements in
supernova explosions was thoroughly tested in February 1987. A
supernova, SN 1987A, exploded in the nearby Large Magellanic Cloud.
Sanduleak—69° 202, which in 1986 was noted as a star of 20 solar
masses, is no longer there. Together the star and the supernova give
dramatic evidence that at least one massive star ended its life in a
violent way.
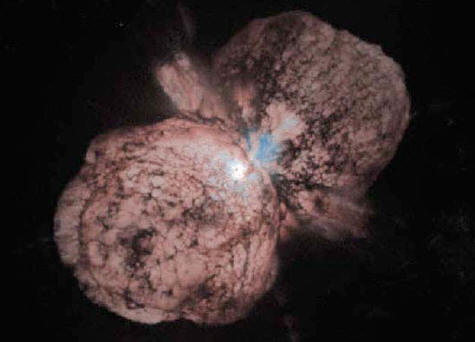
Eta Carinae
Eta Carinae, thought to be at least 150 solar masses, is a supergiant massive star some 7,500 light years from Earth. This star is one of the most luminous star systems in our Galaxy, radiating millions of times more power than our Sun.
Speculation among
astronomers is that Eta Carinae will undergo a supernova explosion
sometime in the next thousand years. But because Eta Carinae is over
the 30 solar mass limit of Type II supernovae, it may be destined to
become a Type Ib/c supernovae associated with ultra luminous
gamma-ray bursters.
Stars destined to become Type II
supernova, such as Sanduleak—69° 202, may also produce similar
discharges.
We have come full circle.
The universe is the evolutionary story of generations; for every death there is a new beginning. In its death throes, supernovae enrich the interstellar medium so that new stars and planets can be born. Every atom of calcium in every bone in our bodies, every atom of iron in our blood, was shot out of a star billions of years ago, before the birth of our own Sun.
We are literally and actually children
of the stars.
Recommended Reading
For more on the origins of the elements on the web go to: For further information on related topics go to:
|