|

by Carl Zimmer
July 12, 2010
from
TheNewYorkTimes Website
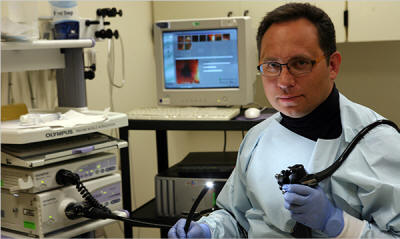
Dr. Alexander Khoruts,
a gastroenterologist at the University Minnesota,
used bacteriotherapy
to help cure a patient suffering from a gut infection.
Dr. Alexander Khoruts had run out
of options.
In 2008, Dr. Khoruts, a gastroenterologist at the University of
Minnesota, took on a patient suffering from a vicious gut infection
of
Clostridium difficile. She was crippled by constant
diarrhea, which had left her in a wheelchair wearing diapers. Dr.
Khoruts treated her with an assortment of antibiotics, but nothing
could stop the bacteria.
His patient was wasting away, losing 60
pounds over the course of eight months.
“She was just dwindling down the
drain, and she probably would have died,” Dr. Khoruts said.
Dr. Khoruts decided his patient needed a
transplant. But he didn’t give her a piece of someone else’s
intestines, or a stomach, or any other organ.
Instead, he gave her
some of her husband’s bacteria.
Dr. Khoruts mixed a small sample of her husband’s stool with saline
solution and delivered it into her colon. Writing in the Journal of
Clinical Gastroenterology last month, Dr. Khoruts and his colleagues
reported that her diarrhea vanished in a day. Her Clostridium
difficile infection disappeared as well and has not returned
since.
The procedure - known as
bacteriotherapy or fecal transplantation -
had been carried out a few times over the past few decades. But Dr. Khoruts and his colleagues were able to do something previous
doctors could not:
they took a genetic survey of the bacteria in her
intestines before and after the transplant.
Before the transplant, they found, her gut flora was in a desperate
state.
“The normal bacteria just didn’t
exist in her,” said Dr. Khoruts. “She was colonized by all sorts
of misfits.”
Two weeks after the transplant, the
scientists analyzed the microbes again. Her husband’s microbes had
taken over.
“That community was able to function
and cure her disease in a matter of days,” said Janet Jansson, a
microbial ecologist at Lawrence Berkeley National Laboratory and
a co-author of the paper. “I didn’t expect it to work. The
project blew me away.”
Scientists are regularly blown away by
the complexity, power, and sheer number of microbes that live in our
bodies.
“We have over 10 times more microbes
than human cells in our bodies,” said George Weinstock of
Washington University in St. Louis.
But the microbiome, as it’s
known, remains mostly a mystery.
“It’s as if we have these other
organs, and yet these are parts of our bodies we know nothing
about.”
Dr. Weinstock is part of an
international effort to shed light on those puzzling organs.
He and his colleagues are cataloging
thousands of new microbe species by gathering their DNA sequences.
Meanwhile, other scientists are running experiments to figure out
what those microbes are actually doing. They’re finding that the
microbiome does a lot to keep us in good health.
Ultimately, researchers hope, they will
learn enough about the microbiome to enlist it in the fight against
diseases.
“In just the last year, it really
went from a small cottage industry to the big time,” said David
Relman of Stanford University.
The microbiome first came to light in
the mid-1600s, when the Dutch lens-grinder Antonie van Leeuwenhoek
scraped the scum off his teeth, placed it under a microscope and
discovered that it contained swimming creatures.
Later generations of microbiologists
continued to study microbes from our bodies, but they could only
study the ones that could survive in a laboratory. For many species,
this exile meant death.
In recent years, scientists have started to survey the microbiome in
a new way: by gathering DNA.
They scrape the skin or take a cheek
swab and pull out the genetic material. Getting the DNA is fairly
easy. Sequencing and making sense of it is hard, however, because a
single sample may yield millions of fragments of DNA from hundreds
of different species.
A number of teams are working together to tackle this problem in a
systematic way.
Dr. Weinstock is part of the biggest of these
initiatives, known as the
Human Microbiome Project. The $150
million initiative was started in 2007 by the National Institutes of
Health. The project team is gathering samples from 18 different
sites on the bodies of 300 volunteers.
To make sense of the genes that they’re gathering, they are
sequencing the entire genomes of some 900 species that have been
cultivated in the lab. Before the project, scientists had only
sequenced about 20 species in the microbiome.
In May, the scientists published details
on the first 178 genomes. They discovered 29,693 genes that are
unlike any known genes. (The entire human genome contains only
around 20,000 protein-coding genes.)
“This was quite surprising to us,
because these are organisms that have been studied for a long
time,” said Karen E. Nelson of the J. Craig Venter Institute in
Rockville, Md.
The new surveys are helping scientists
understand the many ecosystems our bodies offer microbes.
In the mouth alone, Dr. Relman
estimates, there are between 500 and 1,000 species.
“It hasn’t reached a plateau yet:
the more people you look at, the more species you get,” he said.
The mouth in turn is divided up into
smaller ecosystems, like the tongue, the gums, the teeth. Each
tooth - and even each side of each tooth - has a different combination
of species.
Scientists are even discovering ecosystems in our bodies where they
weren’t supposed to exist. Lungs have traditionally been considered
to be sterile because microbiologists have never been able to rear
microbes from them. A team of scientists at Imperial College London
recently went hunting for DNA instead. Analyzing lung samples from
healthy volunteers, they discovered 128 species of bacteria.
Every square centimeter of our lungs is
home to 2,000 microbes.
Some microbes can only survive in one
part of the body, while others are more cosmopolitan. And the
species found in one person’s body may be missing from another’s.
Out of the 500 to 1,000 species of microbes identified in people’s
mouths, for example, only about 100 to 200 live in any one person’s
mouth at any given moment.
Only 13 percent of the species on two
people’s hands are the same. Only 17 percent of the species living
on one person’s left hand also live on the right one.
This variation means that the total number of genes in the human
microbiome must be colossal. European and Chinese researchers
recently catalogued all the microbial genes in stool samples they
collected from 124 individuals. In March, they published a list of
3.3 million genes.
The variation in our microbiomes emerges the moment we are born.
“You have a sterile baby coming from
a germ-free environment into the world,” said Maria
Dominguez-Bello, a microbiologist at the University of Puerto
Rico.
Recently, she and her colleagues studied
how sterile babies get colonized in a hospital in the Venezuelan
city of Puerto Ayacucho.
They took samples from the bodies of
newborns within minutes of birth. They found that babies born
vaginally were coated with microbes from their mothers’ birth
canals.
But babies born by Caesarean section
were covered in microbes typically found on the skin of adults.
“Our bet was that the Caesarean
section babies were sterile, but it’s like they’re magnets,”
said Dr. Dominguez-Bello.
We continue to be colonized every day of
our lives.
“Surrounding us and infusing us is
this cloud of microbes,” said Jeffrey Gordon of Washington
University.
We end up with different species, but
those species generally carry out the same essential chemistry that
we need to survive. One of those tasks is breaking down complex
plant molecules.
“We have a pathetic number of
enzymes encoded in the human genome, whereas microbes have a
large arsenal,” said Dr. Gordon.
In addition to helping us digest, the microbiome helps us in many other ways.
The microbes in our nose, for example,
make antibiotics that can kill the dangerous pathogens we sniff. Our
bodies wait for signals from microbes in order to fully develop.
When scientists rear mice without any germ in their bodies, the mice
end up with stunted intestines.
In order to co-exist with our microbiome, our immune system has to
be able to tolerate thousands of harmless species, while attacking
pathogens. Scientists are finding that the microbiome itself guides
the immune system to the proper balance.
One way the immune system fights pathogens is with inflammation. Too
much inflammation can be harmful, so we have immune cells that
produce inflammation-reducing signals. Last month, Sarkis
Mazmanian and June L. Round at Caltech reported that mice
reared without a microbiome can’t produce an inflammation-reducing
molecule called IL-10.
The scientists then inoculated the mice with a single species of gut
bacteria, known as Bacteroides fragilis. Once the bacteria
began to breed in the guts of the mice, they produced a signal that
was taken up by certain immune cells. In response to the signal, the
cells developed the ability to produce IL-10.
Scientists are not just finding new links between the microbiome and
our health. They’re also finding that many diseases
are accompanied by dramatic changes in the makeup of our inner
ecosystems.
The Imperial College team that discovered microbes in
the lungs, for example, also discovered that people with asthma have
a different collection of microbes than healthy people. Obese people
also have a different set of species in their guts than people of
normal weight.
In some cases, new microbes may simply move into our bodies when
disease alters the landscape. In other cases, however, the microbes
may help give rise to the disease. Some surveys suggest that babies
delivered by Caesarian section are more likely to get skin
infections from multiply-resistant Staphylococcus aureus.
It’s possible that they lack the
defensive shield of microbes from their mother’s birth canal.
Caesarean sections have also been linked to an increase in asthma
and allergies in children. So have the increased use of antibiotics
in the United States and other developed countries. Children who
live on farms - where they can get a healthy dose of microbes from
the soil - are less prone to getting autoimmune disorders than
children who grow up in cities.
Some scientists argue that these studies all point to the same
conclusion:
when children are deprived of their normal supply of
microbes, their immune systems get a poor education.
In some people,
untutored immune cells become too eager to unleash a storm of
inflammation. Instead of killing off invaders, they only damage the
host’s own body.
A better understanding of the microbiome might give doctors a new
way to fight some of these diseases. For more than a century,
scientists have been investigating how to treat patients with
beneficial bacteria. But probiotics, as they’re sometimes called,
have only had limited success.
The problem may lie in our ignorance of
precisely how most microbes in our bodies affect our health.
Dr. Khoruts and his colleagues have carried out 15 more fecal
transplants, 13 of which cured their patients. They’re now analyzing
the microbiome of their patients to figure out precisely which
species are wiping out the Clostridium difficile infections.
Instead of a crude transplant, Dr.
Khoruts hopes that eventually he can give his patients what he
jokingly calls “God’s probiotic” - a pill containing microbes whose
ability to fight infections has been scientifically validated.
Dr. Weinstock, however, warns that a deep understanding of the
microbiome is a long way off.
“In terms of hard-boiled science,
we’re falling short of the mark,” he said.
A better picture of
the microbiome will only emerge once scientists can use the
genetic information Dr. Weinstock and his colleagues are
gathering to run many more experiments.
“It’s just old-time science. There are no short-cuts around
that,” he said.
|

