|

by
Mark Eastman, M.D.
and
Chuck Missler
from
TriunityReport
Website
Contents
Part 1
Despite incredible odds, and seemingly insurmountable problems,
spontaneous
generation is taught as a fact from grammar school to university. |
Stanley Miller's Bombshell
In 1953 a graduate student named Stanley Miller set out to verify the
Oparin-Haldane-Urey
hypothesis with a simple but elegant experiment.1 The results of this
experiment have been taught to every high school and college biology student
for nearly four decades.
Using a system of glass flasks, Miller attempted to simulate the early
atmospheric conditions. He passed a mixture of boiling water, ammonia,
methane and hydrogen through an electrical spark discharge. At the bottom of
the apparatus was a trap to capture any molecules made by the reaction. This
trap prevented the newly-formed chemicals from being destroyed by the next
spark. Eventually, Miller was able to produce a mixture containing very
simple amino acids, the building blocks of proteins.
Miller drew on decades of knowledge of organic chemistry in setting up his
experiment. The proportions of the various gases used, the actual apparatus,
the intensity of the spark and the chemical trap, were all carefully
adjusted to create maximum yield from the experiment.
On the first attempt, after a week of electrical discharges in the reaction
chamber, the sides of the chamber turned black and the liquid mixture turned
a cloudy red. The predominant product was a gummy black substance made up of
billions of carbon atoms strung together in what was essentially tar, a
common nuisance in organic reactions.2 However, no amino acids used by
living systems, or other building blocks of life, were produced on the first
attempt.
After rearranging the apparatus, the experiment produced two amino acids,
glycine and alanine, the simplest amino acids found in living systems. If we
search the remaining products, we find a number of simple amino acids, but
in yields so low that their concentrations would be insignificant in a body
of water.
Table 1. The Products of the Miller Experiment Tar 85%
|
Tar |
85% |
|
Carboxlic acids not important to life
|
13.0% |
|
Glycine |
1.05% |
|
Alanine |
0.85% |
|
Glutamic acid |
trace |
|
Aspartic acid |
trace |
|
Valine |
trace |
|
Leucine |
trace |
|
Serine |
trace |
|
Proline |
trace |
|
Treonine |
trace |
Regarding the products of the Miller-Urey experiment, evolutionist
Robert
Shapiro stated:
"Let us sum up. The experiment performed by
Miller yielded tar as its most
abundant product....There are about fifty small organic compounds that are
called 'building blocks'.....Only two of these fifty occurred among the
preferential Miller-Urey products."
3
In the past forty years, many scientists have repeated the work of
Miller
and Urey. Electrical sparks, heat, ultraviolet radiation, light, shock waves,
and high energy chemical catalysts have been used in an attempt to create
the building blocks of life.
4 In general, when amino acids have been made,
they occur in approximately the same proportion, with glucine and
alanine
predominating, as in the Miller's experiment.
The Case of the Missing Letters
In the English language convention there are twenty-six letters that are
used to write sentences, paragraphs, chapters, and books. These letters are
strung together according to hundreds of predetermined rules. Anyone with a
knowledge of those rules can understand the information conveyed by the
sequence of letters.
In all living systems there are a special set of four chemical "letters,"
called nucleotides, which are used to "write" the information stored by the
code of life, the Genetic Code. Millions of these nucleotides are strung
together, end to end, in long chains, thus forming the DNA molecule (Figure
1). The instructions necessary to produce all the living structures on earth
are "written" by the rules of the genetic code and carried by these chains
of chemical letters. These chemical letters represent only a tiny part of
the "hardware" that must arise by chance in order for
spontaneous generation
to occur. However, nucleotides are much more complex than the simple amino
acids made by Miller and Urey, and would require much more chemical
expertise to produce.
Many claims have been made that nucleotides of DNA have been produced in
such "spark and soup" experiments. However, after a careful review of the
scientific literature, evolutionist Robert Shapiro stated that the
nucleotides of DNA and RNA,
"....have never been reported in any amount in such sources, yet a mythology
has emerged that maintains the opposite....I have seen several statements in
scientific sources which claim that proteins and nucleic acids themselves
have been prepared... These errors reflect the operation of an entire belief
system...The facts do not support his belief... Such thoughts may be
comforting, but they run far ahead of any experimental validation."
5 (Emphasis
added).
|
Figure 1
DNA
Deoxyribonucleic Acid
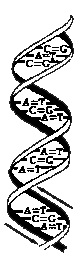
| |
|
Nucleotides |
| |
|
A= Adenine |
| |
|
T= Thymidine |
| |
|
C= Cytosine |
| |
|
G= Guanine |
The DNA molecule is formed by two chains of nucleotides which are bonded together to form the structure of a spiral double helix. Somewhat like a ladder which is twisted from the top down.
|
After nearly four decades of trying, with the best equipment and the best
minds in chemistry, not even the "letters" of the genetic code have been
produced by random chemical processes. If the letters cannot be produced by
doctorate-level chemists, how can we logically assume that they arose by
chance in a chemical quagmire?
A Troubled Paradigm
Stanley Miller's experiment was seen by believers as virtual proof that
organic chemicals, and ultimately life, could be produced by chance
chemistry. It brought a greater measure of scientific respectability to the
theory of spontaneous generation and evolutionary thought. Evolution,
according to the purists, could now be taught as a virtual certainty. The
impact of this experiment on the scientific community is expressed by
evolutionist and astronomer Carl Sagan:
"The Miller-Urey experiment is now recognized as the single most significant
step in convincing any scientists that life is likely to be abundant in the
cosmos."
6
This opinion, however, is not universally held by evolutionists. With the
advantage of three decades of hindsight, and extensive discoveries in
molecular biology, evolutionist Robert Shapiro comments on the significance
of the Miller-Urey experiments:
"The very best
Miller-Urey chemistry, as we have seen, does not take us very
along the path to a living organism. A mixture of simple chemicals, even one
enriched in a few amino acids, no more resembles a bacterium than a small
pile of real and nonsense words, each written on an individual scrap of
paper, resembles the complete works of Shakespeare."
7
After a careful examination of the
Miller experiment, Shapiro recognized
that the simple chemicals he produced are a far cry from the incredible
complexity of a living cell.
In the last 20 years a number of scientists have spoken out regarding the
problems with the Haldane-Oparin paradigm. Most of the assumptions of the
primordial atmosphere, even the existence of the "primordial soup," have
been seriously questioned by origins researchers. Carl Woese, of the
University of Illinois expressed the inadequacy of the Oparin thesis:
"The Oparin thesis has long ceased to be a productive paradigm: it no longer
generates novel approaches to the problem... These symptoms suggest a
paradigm whose course is run, one that is no longer a valid model of the
true state of affairs."
8
Let's look at some of the evidence that has threatened the
Oparin-Haldane-Miller thesis.
THE MYTH OF THE PRE-BIOTIC ATMOSPHERE
The Oxygen Problem
The atmospheric conditions proposed by Oparin, Haldane and Urey were
radically different from what presently exists. Because oxygen destroys the
chemical building blocks of life, they speculated that the early earth had
an oxygen-free atmosphere. However, in the last twenty years, evidence has
surfaced that has convinced most atmospheric scientists that the early
atmosphere contained abundant oxygen.
In the 1970's Apollo 16 astronauts discovered that water is broken down into
oxygen and hydrogen gas in the upper atmosphere when it is bombarded by
ultraviolet radiation. This process, called photo dissociation, is an
efficient process which would have resulted in the production of large
quantities of oxygen in a relatively short time. Studies by the astronauts
revealed that this process is probably a major source of oxygen in our
current atmosphere.
2 H2O + uv Radiation -- H2 (hydrogen gas) + O2 (oxygen
gas)
The assumption of an oxygen-free atmosphere has also been rejected on
theoretical grounds. The ozone layer around planet earth consists of a thin
but critical blanket of oxygen gas in the upper atmosphere. This layer of
oxygen gas blocks deadly levels of ultraviolet radiation from the sun.
9
Without oxygen in the early atmosphere, there could have been no ozone layer
over that early earth. Without an ozone layer, all life on the surface of
planet earth would face certain death from exposure to intense ultraviolet
radiation. Furthermore, the chemical building blocks of proteins, RNA
and DNA, would be quickly annihilated because ultraviolet radiation destroys
their chemical bonds.
10 It doesn't matter if these newly formed building
blocks are in the atmosphere, on dry ground, or under water.11,12,13
So we have a major dilemma. The products of the Miller-Urey experiments
would be destroyed if oxygen was present, and they would be destroyed if it
wasn't! This "catch 22" has been noted by evolutionist and molecular
biologist Michael Denton:
"What we have then is a sort of
'Catch 22' situation. If we have oxygen we
have no organic compounds, but if we don't we have none either."
14
Even if the building blocks of life could survive the effects of intense
ultraviolet radiation and form life spontaneously, the survival of any
subsequent life forms would be very doubtful in the presence of such heavy
ultraviolet light. Ozone must be present to protect any surface life from
the deadly effects of ultraviolet radiation from the sun.
Finally, the assumption that there was no oxygen in the early atmosphere is
not borne out by the geologic evidence. Geologists have discovered evidence
of abundant oxygen content in the oldest known rocks on earth. Again,
Michael Denton:
"Ominously, for believers in the traditional organic soup scenario, there is
no clear geochemical evidence to exclude the possibility that oxygen was
present in the Earth's atmosphere soon after the formation of its crust."
15
All of this evidence supports the fact that there was abundant oxygen on the
early earth.
Ammonia and Methane Short Lived
The assumption of an atmosphere consisting mainly of ammonia, methane,
and hydrogen, has also been seriously questioned. In the 1970's scientists
concluded that ultraviolet radiation from the sun, as well as simple
"rainout," would eliminate ammonia and methane from the upper atmosphere in
a very short time.16 In 1981, Atmospheric scientists from
NASA concluded
that:
"the methane and ammonia-dominated atmosphere would have been very short
lived, if it ever existed at all."
17
The Myth of the Pre-biotic Soup
During the last two decades, the notion of a primordial soup has not fared
too well either. Studies of the atmosphere, ultraviolet radiation, and the dilutional effect of a large body of water, have convinced many scientists
that the ocean could not have developed into the "hot dilute soup" that was
envisioned by Darwin, Oparin, and Haldane.
Oparin envisioned the production of cellular building blocks in the
atmosphere as a result of lightning or ultraviolet radiation. Stanley
Miller's experiment attempted to validate this concept. Once produced, these
chemicals would theoretically build up in the primordial oceans and combine
to form the first living systems. However, since Miller's experiments in
1953, it has been estimated that it would take up to two years for amino
acids to fall from the atmosphere into the ocean.
18 This is a problem
because even small amounts of ultraviolet radiation would destroy the
building blocks before they reached the oceans. Furthermore, as we saw
earlier, lack of ozone would further expedite this destruction.
19
Saved By The Trap!
A problem seldom noted by textbooks is that the chemical reactions that
produced the amino acids in Miller's experiments are reversible. That is,
the same energy sources that cause the formation of the building blocks of
life will also destroy those same building blocks unless they are removed
from the environment where they were created. In fact, the building blocks
of life are destroyed even more efficiently than they are created. This was
foreseen by Miller and Urey, so they included a chemical trap to remove the
newly formed chemicals before the next spark. Of course, this luxury would
not be available on the early earth.
These problems have convinced many origins researchers that the idea of a
primordial soup is quite unlikely. Michael Denton comments on the lack of
evidence for the primordial soup:
"Rocks of great antiquity have been examined over the past two decades and
in none of them has any trace of abiotically produced organic compounds been
found...Considering the way the pre- biotic soup is referred to in so many
discussions of the origin of life as an already established reality, it
comes as something of a shock to realize that there is absolutely no
positive evidence for its existence."
20 (Emphasis added).
The Origin of DNA and Proteins
Up to this point we've discussed the origin of just the building blocks of
living cells. The destructive effect of,
-
oxygen,
-
ultraviolet radiation from
the sun
-
and the short duration of an optimal atmosphere for their
production,
makes it unlikely that significant quantities of viable
nucleotides and amino acids could ever accumulate in the primitive ocean.
However, even if they did accumulate in sufficient quantities, the next step
is to explain how they combined to form the self-duplicating DNA molecule
and the thousands of proteins found in the simplest living cells. For the
materialistic scenario to be taken seriously, it must provide a plausible
explanation for the origin of these enormous molecules without the
introduction of biochemical know-how.
The Problem of Chirality
One of the most difficult problems for the materialistic scenario on the
origin of life is something called molecular chirality. The building blocks
of DNA and proteins are molecules which can exist in both right and
left-handed mirror-image forms (Figure 2). This "handedness" is called
"chirality."
21,
22
These mirror-image chemicals are referred to as
dextrorotary (dextro-form) and levorotary (levo-form).
23
|
Figure 2
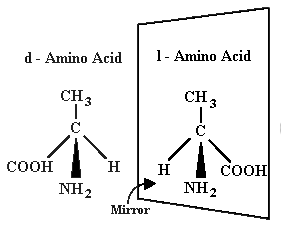
Levo and Dextro Amino Acids
|
In all living systems the building blocks of the DNA and
RNA exist
exclusively in the right-handed form, while the amino acids in virtually all
proteins in living systems, with very rare exception, occur only in the left-handed form.
24
The dilemma for materialists is that all "spark and soup-like" experiments
produce a mixture of 50% left (levo) and 50% right-handed
(dextro)
products.
25,
26 Such a mixture of
dextro and levo amino acids is called a
"racemic mixture." Unfortunately, such mixtures are completely useless for
the spontaneous generation of life.
27
Complex molecules such as DNA and proteins are built by adding one building
block at a time onto an ever-growing chain. In a "primordial soup" made up
of equal proportions of right and left-handed building blocks, there is an
equal probability at each step of adding either a right or left-handed
building block.
28,
29 Consequently, it is a mathematical absurdity to propose
that only right-handed nucleotides would be added time after time without a
single left-handed one being added to a growing DNA molecule. Sooner or
later an incorrect, left-handed nucleotide will be added. The same goes for
proteins. Every time another amino acid is added to the growing chain of
amino acids the chances are virtually certain that both right and
left-handed amino acids will be added.
With unguided or undirected chemistry, a primordial ooze consisting of right
and left-handed building blocks can only result in the production of
DNA and proteins composed of a mixture of right and left-handed building blocks.
This dilemma has enormous implications for the materialistic scenario.
30 For
a living cell to function properly, it is absolutely necessary for it to
contain the correct three-dimensional structure in its DNA and
proteins.
This correct three-dimensional structure is in turn dependent upon proteins
built from a pure mixture of left-handed amino acids and
DNA built from
right-handed nucleotides. Consequently, if even one nucleotide or amino acid
with the incorrect "handedness" is inserted into a DNA or
protein molecule, the three-dimensional structure will be annihilated and it will cease to
function normally.
Enzymes: The Cell's Miniature Factories
The importance of the three-dimensional structure of proteins can best be
illustrated by the function of enzymes. Virtually all of the complex
chemical reactions in living cells involve special proteins called
enzymes.
Enzymes act to speed up (catalyze) chemical reactions in biological systems.
Enzymes are employed in the production of DNA,
RNA, proteins, and nearly
every chemical reaction in the cell. Digestion, thought, sight, and the
function of nerve and muscles all require the use of enzymes. In fact, these
activities would be impossible without them.
Enzymatic reactions occur like "lock and key" mechanisms. An
enzyme (the
lock) has a highly specific three-dimensional shape which will only allow
chemicals with the correct three-dimensional fit (the key) to bind and
result in a chemical reaction. (Figure 3).
|
Figure 3
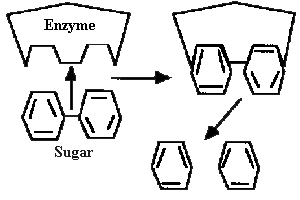
In this illustration the enzyme breaks the bond that holds two sugar molecules together releasing two unbonded sugars. |
The three-dimensional structure of these protein enzymes (which is
determined by the sequence of pure l-amino acids) must be preserved within a
narrow range or these "lock and key" chemical reactions cannot occur.
Consequently, a primordial soup consisting of equal portions of left and
right-handed amino acids, which will only result in proteins containing
equal portions of left and right-handed amino acids, is incapable of forming
enzymes with the correct three-dimensional shapes and precise "lock and key"
mechanisms. Therefore, a primordial soup of left and right-handed building
blocks is completely incapable of forming life.
Since all spark and soup experiments produce a 50/50 mix of right and
left-handed amino acids, chemists have tried to decipher how only
left-handed amino acids became integrated into the proteins of living
systems. For decades chemists have attempted to separate out a pure mixture
of left-handed amino acids from a racemic mix by chance chemistry alone.
Chance, or un-directed chemistry has, however, consistently proven to be an
inadequate mechanism for the separation of the right and left-handed amino
acid forms.
31 So, how did it happen? Mathematically, random-chance would
never select such an unlikely pure molecule out of a racemic primordial
soup.
The solution is simple, yet it has profound implications. To separate the
two amino acid forms requires the introduction of biochemical expertise or
know-how, which is the very antithesis of chance! However, biochemical
expertise or know-how comes only from a mind. Without such know-how or
intelligent guidance, the right and left-handed building blocks of life will
never separate. Consequently, enzymes, with their lock and key mechanisms,
and ultimately, life, are impossible!
32
However, the existence of a mind or a Creator involved in the creation of
life is anathema to the atheist's scenario. But the volume of biochemical
knowledge supports this fact: To produce pure mixtures of left-handed amino
acids and right-handed nucleotides, requires intelligent guidance.
And since
no human chemists were around before the origin of life on earth, the source
of this intelligent guidance must have been extraterrestrial!
Toxic Waste Wipes Out Spontaneous Generation
The major products made in Miller's experiment were a mixture of tar and
thousands of organic acids. This "chemical junk," which comprised 98% of the
material produced by Miller, is very similar to the chemical waste that the
U.S. government is spending billions of dollars to remove from neighborhoods
all around the country. Why are they removing these chemicals? Because they
are toxic to humans.
Organic acids, such as those produced by Miller, can damage
DNA, causing
cancer and other diseases. They also poison our enzymes by irreversibly
binding to them.
33 Any primordial soup would be filled with these toxic
products and would quickly and efficiently prevent the functioning of
DNA, RNA, and proteins. The result: death! In fact, it is unlikely that any
currently living cell on earth could survive in the chemical environment
produced by Miller's experiment.
34 Considering the toxicity of the
primordial soup, it is perhaps the last place on earth that life might
arise.35
H2O "Washes Up" Spontaneous Generation
We noted previously that DNA and proteins are built by adding one building
block at a time onto an ever-lengthening chain. With the addition of each
amino acid or nucleotide, a molecule of water is released. This is called a
condensation reaction and is fully reversible, i.e., it can proceed in
either direction as indicated by the arrows in figure 4.
|
Figure 4
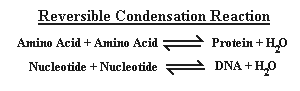
|
In previous sections we have seen that neither air nor land are safe havens
for the newly formed building blocks of life because of their certain
destruction by oxygen or intense UV radiation. So believers in
spontaneous
generation have concluded that the first life forms may have arisen near a
deep sea volcanic vent, safe from oxygen and UV radiation. Although a water
environment may seem like safe place for the formation of life, it is the
release of a water molecule in the above reaction that creates one of the
most difficult problems for the theory of spontaneous generation.
Every first-year chemistry student is taught that reversible chemical
reactions will never proceed in a direction that produces a product that is
already present in excess amounts in the reaction vessel.
36
The production
of DNA and proteins from their building blocks results in the production of
a large number of water molecules. A problem for the oceanic vent theory (or
any water based primordial soup theory) is that there is already an
abundance of water. Consequently, the reaction above will never proceed in a
direction which produces more water. In fact, the laws of chemistry and
thermodynamics demand that the reaction go in the opposite direction!
Therefore, in a watery solution containing the building blocks of life, the
overwhelming majority of these building blocks would be unbonded. As a
result, a watery environment is perhaps the last place that long chains of
amino acids or nucleotides would form.
37
Equilibrium - The Villain of the Plot
There is one final hurdle that must be successfully cleared if the
materialist's scenario on the origin of life is to have credibility. This is
the problem of chemical equilibrium. The notion of equilibrium is one with
which you are all familiar, even if you've never taken a chemistry course.
In any broth or solution we notice that there is the tendency for the
materials to become evenly distributed with time. This tendency is called
the development of equilibrium.
38
A simple example will help us to understand. If a drop of red dye is put
into a container of water the dye particles gradually disperse throughout
the solution until the entire solution turns a dilute red color. The larger
the volume of the solvent (i.e., the water in our dye experiment), the more
dilute will be the solution once the dye particles have become evenly
distributed. This dilutional effect is irreversibly tied to the arrow of
time. As time advances, as predicted by the Second Law,
the dye particles
become evenly distributed until the solution reaches a state of chemical
equilibrium.
39
As we saw previously, the chemical reactions leading to the formation of
DNA and proteins are reversible. This means that the building blocks of
DNA and proteins are broken off of the chain just as easily as they are added.
Consequently, the building blocks of life, if they survived the effects of
oxygen and UV radiation, would constantly be combining and coming apart in
the primordial soup. This combining and coming apart of chemical building
blocks proceeds until a state of equilibrium is reached. In the case of
amino acids and nucleotides, the building blocks of
DNA and proteins will be
predominantly unbonded when the solution is at equilibrium.
40,
41
Since the natural tendency for the building blocks of life is to disperse
and remain un-bonded, the question materialists must answer is how did the
building blocks of life become bonded and stay bonded in a primordial soup
which is steadily progressing towards equilibrium?
In living systems enzymes are "programmed" to accomplish this feat by
extracting and utilizing energy from the environment to synthesize and
preserve DNA and proteins.
42
Consequently, in this capacity enzymes fulfill
the definition of a machine or an engine, as defined by Nobel Laureate Jaques Monod - a purposeful (teleonomic) aggregate of matter that uses
energy to perform work.
In the absence of such molecular machinery (i.e., enzymes), the
reversibility of these chemical reactions ensures that any building blocks
which may have become bonded will rapidly become unbonded in a watery
environment unless they are removed from the solution in equilibrium.
43,
44
However, removing the building blocks from equilibrium
requires a mechanism
or a metabolic machine (which do not arise by chance).
Harold Blum dealt with this very dilemma. He recognized that the production
of proteins or DNA from a solution of unbonded building blocks required a
"mechanism" or metabolic "motor" that can capture free energy from the
environment, then use it to remove the building blocks from equilibrium,
i.e. keep them bonded:
"...If proteins were reproduced as they must have been, if living systems
were to evolve - free energy has to be supplied. The source of this free
energy is a fundamental problem we must eventually face...the fact remains
that no appreciable amounts of polypeptides [proteins] would form unless
there were some factor which altered the equilibrium greatly in their
favor."
45
By altering "the equilibrium greatly in their favor," Blum means
allowing
them to stay bonded. However, inanimate matter contains no "mechanism,"
"machines," or "biochemical know-how" that can extract free energy from the
environment and store or preserve the bonded building blocks before they
break down again.
Therefore, the dilemma for the materialist is explaining the origin of the
first such metabolic "machine" by chance. In practice and in theory,
machines are never the result of chance. They are the result of design.
46,
47
This fact is not only intuitive, but it has been verified by the
overwhelming body of experimental science.
A.E. Wilder-Smith addresses this problem of the origin of the first
metabolic motor:
"What Dr. Blum is saying is: how was the motor to extract the energy from
the environment built before life processes had arisen to build it? Once a
motor (enzyme metabolic system) is present, it can easily supply the free
energy necessary to build more and more motors, that is, to reproduce. But
the basic problem is: How do we account for the building of the first
complex enzymatic protein metabolic motor to supply energy for reproduction
and other cell needs....The Creationist believes that God synthesized
non-living matter into living organisms and thus provided the motors which
were then capable of immediately extracting energy from their environment to
build more motors for reproduction. This view is thus perfectly sound
scientifically and avoids the hopeless impasse of the materialistic.
Darwinists in trying to account for the design and building of the first
necessarily highly complex metabolic motors by random processes. Once the
motor has been designed, fabricated, and is running, the life processes work
perfectly well on the principles of the known laws of thermodynamics...."
48
So the net of this dilemma is that intelligent guidance is required to
create a metabolic motor which will synthesize and preserve the chains of
DNA and proteins. Such guidance comes only from
a mind, and not from
inanimate inorganic matter!
Time: The Unlikely Villain
When confronted with the problem of equilibrium, most scientific
materialists will appeal to the magic ingredient of time. In chapter one we
saw this appeal by Nobel Laureate, George Wald:
"Time is in fact the hero of the plot. Given so much time the impossible
becomes possible, the possible probable, and the probable virtually certain.
One has only to wait: Time itself performs the miracles."
49
However, Dr. Blum, who is a materialist, points out that Wald's
faith in the
miraculous ingredient of time is mere wishful thinking. Prolonged time
periods, he asserts, actually worsen the dilemma:
"I think if I were rewriting this chapter [on the origin of life]
completely, I should want to change the emphasis somewhat. I should want to
play down still more the importance of the great amount of time available
for highly improbable events to occur. One may take the view that the
greater the time elapsed the greater should be the approach to equilibrium,
the most probable state, and it seems that this ought to take precedence in
our thinking over the idea that time provides the possibility for the
occurrence of the highly improbable."
50 (Emphasis added)
According to Dr. Blum, the magic bullet of time does not increase the
likelihood that chains of DNA or proteins will form by chance chemistry. In
fact, according to Dr. Blum, increasing the time factor actually ensures
that any primordial soup would consist of predominantly unbonded amino
acids and nucleotides!
The Chicken or the Egg?
Any discussion of the origin of life would not be complete without a look at
the greatest paradox of all:
What came first, DNA or the proteins essential
for the production of DNA?
Since the structure of DNA was deciphered in 1953, biologists have
discovered that the process of duplicating DNA requires as many as twenty
specific protein enzymes. These enzymes function to unwind, un-zip, copy,
and rewind the DNA molecule. There are even enzymes that screen and correct
for copying errors!
The instructions for the production of all proteins, including these
enzymes, are in turn stored on the DNA molecule. So which came first: The
DNA molecule or the proteins necessary to make DNA? You can't make DNA
without highly specific proteins. But you can't make proteins unless you
have a system in place to code for and build those proteins in the first
place. And that means DNA.
Harold Blum recognized this catch 22 when he stated:
"...The riddle seems to be: How, when no life existed, did substances come
into being which, today, are absolutely essential to living systems, yet
which can only be formed by those systems?... A number of major properties
are essential to living systems as we see them today, the origin of any of
which from a 'random' system is difficult enough to conceive, let alone the
simultaneous origin of all."
51
Robert Shapiro also commented on this dilemma:
"Genes and enzymes are linked together in a living cell - two interlocked
systems, each supporting the other. It is difficult to see how either could
manage alone. Yet if we are to avoid invoking either a Creator
or a very
large improbability, we must accept that one occurred before the other in
the origin of life. But which one was it? We are left with the ancient
riddle: Which one came first, the chicken or the egg?"
52
The simultaneous origin of DNA, RNA, and the proteins necessary to produce
them is, according to Blum and Shapiro, very difficult to conceive. In fact,
as we will see next, it is a mathematical impossibility.
The Odds
During the last several decades a number of prestigious scientists have
attempted to calculate the mathematical probability of the random-chance
origin of life. The results of their calculations reveal the enormity of the
dilemma faced by materialists.
In the 1950's Harold Blum estimated the probability of just a single protein
arising spontaneously from a primordial soup. Equilibrium and the
reversibility of biochemical reactions eventually led Blum to state:
"The spontaneous formation of a polypeptide of the size of the smallest
known proteins seems beyond all probability. This calculation alone presents
serious objection to the idea that all living matter and systems are
descended from a single protein molecule which was formed as a 'chance'
act."
53
In the 1970's British astronomer Sir Frederick Hoyle set out to calculate
the mathematical probability of the spontaneous origin of life from a
primordial soup environment. Applying the laws of chemistry, mathematical
probability and thermodynamics, he calculated the odds of the spontaneous
generation of the simplest known free-living life form on earth - a
bacterium.
Hoyle and his associates knew that the smallest conceivable free-living life
form needed at least 2,000 independent functional proteins in order to
accomplish cellular metabolism and reproduction. Starting with the
hypothetical primordial soup he calculated the probability of the
spontaneous generation of just the proteins of a single amoebae.
54
He
determined that the probability of such an event is one chance in ten to the
40 thousandth power, i.e., 1 in 1040,000. Prior to this project, Hoyle was a
believer in the spontaneous generation of life. This project, however,
apparently changed his opinion 180 degrees.
Mathematicians tell us that if an event has a probability which is less
likely than one chance in 1050, then that event is mathematically
impossible. Such an event, if it were to occur, would be considered a
miracle.
Consider this. To win a state lottery you have about 1 chance in ten million
(10/7). The odds of winning the state lottery every single week of your life
from age 18 to age 99 is 1 chance in 4.6 x 1029,120. Therefore, the odds of
winning the state lottery every week consecutively for eighty years is more
likely than the spontaneous generation of just the proteins of an amoebae!
In his calculations Hoyle assumed that the primordial soup consisted
only of
left-handed amino acids. As we noted before, spark and soup-type experiments
always yield a 50/50 mix of left and right-handed building blocks. Hoyle
knew that if the soup consisted of equal portions of right and left-handed
amino acids then mathematical probability of the origin of pure left-handed
proteins would be exactly zero!
After completing his research, Hoyle stated that the probability of the
spontaneous generation of a single bacteria, "is about the same as the
probability that a tornado sweeping through a junk yard could assemble a 747
from the contents therein.
55
Hoyle also stated:
"The likelihood of the formation of life from inanimate matter is one to a
number with 40 thousand naughts [zeros] after it. It is enough to bury
Darwin and the whole theory of evolution. There was no primeval soup,
neither on this planet nor on any other, and if the beginnings of life were
not random they must therefore have been the product of purposeful
intelligence."
56 (Emphasis added)
Hoyle's calculations may seem impressive, but they don't even begin to
approximate the difficulty of the task. He only calculated the probability
of the spontaneous generation of the proteins in the cell. He did not
calculate the chance formation of the DNA, RNA, nor the cell wall that holds
the contents of the cell together.
A more realistic estimate for spontaneous generation has been made by Harold Morowitz, a Yale University physicist.
57
Morowitz imagined a broth of living
bacteria that were super-heated so that all the complex chemicals were
broken down into their basic building blocks. After cooling the mixture, he
concluded that the odds of a single bacterium re-assembling by chance is one
in 10100,000,000,000. This number is so large that it would require several
thousand blank books just to write it out. To put this number into
perspective, it is more likely that you and your entire extended family
would win the state lottery every week for a million years than for a
bacterium to form by chance!
In his book, Origins-A Skeptics Guide to the Creation of Life on Earth,
Robert Shapiro gives a very realistic illustration of how one might estimate
the odds of the spontaneous generation of life. Shapiro begins by allowing
one billion years (5 x 1014 minutes) for spontaneous biogenesis. Next he
notes that a simple bacterium can make a copy of itself in twenty minutes,
but he assumes that the first life was much simpler. So he allows each trial
assembly to last one minute, thus providing 5 x 1014 trial assemblies in 1
billion years to make a living bacterium.
Next he allows the entire ocean to
be used as the reaction chamber. If the entire ocean volume on planet earth
were divided into reaction flasks the size of a bacterium we would have
10/36 separate reaction flasks. He allows each reaction flask to be filled
with all the necessary building blocks of life. Finally, each reaction
chamber is allowed to proceed through one-minute trial assemblies for one
billion years. The result is that there would be 1051 tries available in 1
billion years. According to Morowitz we need 10100,000,000,000 trial
assemblies!
Regarding the probabilities calculated by Morowitz, Robert Shapiro wrote:
"The improbability involved in generating even one bacterium is so large
that it reduces all considerations of time and space to nothingness. Given
such odds, the time until the black holes evaporate and the space to the
ends of the universe would make no difference at all. If we were to wait, we
would truly be waiting for a miracle."
58
Regarding the origin of life Francis Crick, winner of the Nobel Prize in
biology, stated in 1982:
"An honest man, armed with all the knowledge available to us now, could only
state that in some, the origin of life appears at the moment to be almost a
miracle, so many are the conditions which would have had to have been
satisfied to get it going."
59
Regarding the probability of spontaneous generation, Harvard University
biochemist and Nobel Laureate, George Wald stated in 1954:
"One has to only contemplate the magnitude of this task to concede that the
spontaneous generation of a living organism is impossible. Yet we are
here-as a result, I believe, of spontaneous generation."
60
In this incredible statement by Wald we see that his adherence to the
materialist's paradigm is independent of the evidence. Wald's belief in the
"impossible" can only be explained by faith: "...the substance of things
hoped for, the evidence of things not seen."
61
Despite these incredible odds, and the seemingly insurmountable problems we
have discussed, spontaneous generation is taught as a fact from grammar
school to university. In fact, NASA scientists reported to the press in 1991
their opinion that life arose spontaneously not once, but multiple times,
because previous attempts were wiped out by cosmic catastrophes!
The reason for this fanatical adherence to spontaneous generation is
eloquently pointed out by George Wald:
"When it comes to the origin of life there are only two possibilities:
Creation or spontaneous generation. There is not third way.
Spontaneous generation was disproved one hundred years ago, but that leads
us to only one other conclusion, that of supernatural creation. We cannot
accept that on philosophical grounds; therefore, we choose to believe the
impossible: That life arose spontaneously by chance!"
62 (Emphasis added)
According to Wald, it's not a matter of the evidence, it's a
matter of
philosophy! Like George Wald, many people do not like, and cannot accept the
alternative: that all life on earth was created by a transcendent Creator.
So, as Wald said, they are willing to "believe the impossible," in order to
cling to their belief that the universe is a closed system. A system that
has no room for such a Creator.
Man A Machine!! Paley Vindicated
When William Paley put forth his watchmaker argument in 1818, the force of
his argument was weakened by David Hume's assertion that the "machine"
analogy was only superficial. Hume argued that the analogy between machines
and living systems could not be shown to extend to the "deepest" (molecular)
level. Therefore, according to Hume, the analogy was invalid and there was
no need for a designer for biological systems.
During the time of Darwin and Hume, the living cell was viewed as a mere
blob of amorphous unorganized protoplasm. Consequently, Hume's assertion
that the cell was not "machine-like" seemed reasonable. For nearly 150 years
Paley's watchmaker argument was felt to be fatally weakened by the reasoning
of Hume.
However, the astonishing discoveries in molecular biology during the last 40
years have finally and unequivocally demonstrated that living systems are,
in fact, machines - even to the deepest, molecular level! From the tiniest
enzyme to the most complex organ systems found in man, Paley's machine
analogy is confirmed.
At the enzymatic level we see an eerie resemblance to the design and
operation of chemical factories. At the organ level we find "hardware" of an
unimaginable complexity and ingenuity. In our five senses we find sensory
receivers made of multiple components, each machine-like, the operation of
which is absolutely necessary for each sense (taste, sight, smell, hearing,
touch) to function properly. In the function of the human heart we see an
incredibly efficient and durable hydraulic pump, the likes of which no
engineer has imagined. Finally, in the structure of the human brain we find
a computer 1000 times faster than a Cray supercomputer with more connections
than all the computers, phone systems and electronic appliances on planet
earth!
In each of these systems, at every level, we find machine-like structures
which are truly "teleonomic" (purposeful) aggregates of matter, each
executing its role in a pre-programmed manner.
In 1985 evolutionist Michael Denton made this astonishing admission
regarding Paley's machine analogy:
"It has only been over the past twenty years with the molecular biological
revolution and with the advance in cybernetic and computer technology that
Hume's criticism has been finally invalidated and the analogy between
organisms and machines has at last become convincing... In every direction
the biochemist gazes, as he journeys through this weird molecular labyrinth,
he sees devices and appliances reminiscent of our own twentieth-century
world of advanced technology. We have seen a world as artificial as our own
and as familiar as if we had held up a mirror to own machines... Paley was
not only right in asserting the existence of an analogy between life and
machines, but was also remarkably prophetic in guessing that the
technological ingenuity realized in living systems is vastly in excess of
anything yet accomplished by man."
63 (Emphasis added)
The implication of vindicating Paley's machine analogy were also noted by
Denton:
"If we are to assume that living things are machines for the purpose of
description, research and analysis, and for the purposes of rational and
objective debate, as argued by Michael Polyani and Monod among many others,
there can be nothing logically inconsistent, as Paley would have argued, in
extending the usefulness of the analogy to include an explanation for their
origin."
64
Since machines need a designer and since living systems possess "appliances
reminiscent of our own twentieth-century world of advanced technology" it is
"logically" consistent to assert that such appliances (the mechanisms in
living systems) must, according to Denton, require a designer as well!
Consequently, according to Denton:
"The conclusion may have religious implication."
65
Finally, consider this provocative statement by Hoyle and Wickramasinghe:
"The speculations of The Origin of Species
turned out to be wrong...It is
ironic that the scientific facts throw Darwin out, but leave William Paley,
a figure of fun to the scientific world for more than a century, still in
the tournament with a chance of being the ultimate winner."
66
If the most knowledgeable chemists using the most up to date equipment
cannot create machines as complex as a single amoebae, is it credible to
assert that chance, which is the antithesis of intelligence or know-how
could do so? I think not.
The Emperor is naked-and many in the scientific establishment are beginning
to suspect!
Back to Contents
Footnotes:
1. Stanley Miller, Science, Vol 117,(1953).pp. 528-529.
2. The remaining 15% of the reaction products consisted of thirteen organic
chemicals in concentrations ranging from .25% to 4%. All of the thirteen
products were in a class of chemicals known as carboxylic acids. Amino
acids, the building blocks of proteins are one type of carboxylic acid.
There are an unlimited number of carboxylic acids that could be made. The
smallest carboxylic acid possible is formic acid, with only one carbon atom,
and in fact, was the most prominent carboxylic acid made with a yield of 4%.
This acid is unimportant in most life forms, a although it is found in ant
venom! Three other carboxylic acids, with three carbon atoms, but
unimportant to life, were made with a yield of 2.7%.
3. Robert Shapiro, Origins-A Skeptics Guide to the Creation of Life on
Earth,(1986),pg. 105.
4. This body of work is detailed in the book The Mystery of Life's Origin.
C. Thaxton, W. Bradley, R. Olsen, Chapter 3.
5. Shapiro, op. cit., 108-109.
6. Shapiro, op. cit., 99.
7. Shapiro, op. cit., 116.
8. Shapiro, op. cit., 114.
9. Ozone, which consists of three oxygen atoms bonded together, is made when
oxygen in the atmosphere interacts with ultraviolet radiation from the sun.
O2 + Ultraviolet Light = Ozone (O3).
10. Eventually, exposure to ultraviolet radiation will break down amino
acids down into tar, water, methane, and ammonia.
11. Ultraviolet radiation of this intensity would wipe out all newly formed
building blocks even to a depth of ten meters under the water.
12. It has been estimated that in order to form an effective ozone layer,
the atmospheric oxygen content would need to be at least 10% of the amount
in our current atmosphere. However, this same concentration of oxygen is
also enough to quickly and effectively wipe out those same building blocks.
Ultraviolet light breaks the chemical bonds of complex molecules such as
amino acids and nucleotides, making them useless for the spontaneous
generation of life.
13. C. Thaxton, W. Bradley, R. Olsen, The Mystery of Life's Origin; Chapter
3.
14. Denton, op. cit., 262.
15. Denton, op. cit., 261.
16. Rainout means the effect that simple rain would have on the
concentrations of atmospheric methane and ammonia. In a very short time,
rain alone would eliminate most of these substances from the early
atmosphere.
17. Joel Levine, and Tommy Augustson, The Pre biological Paleoatmosphere:
Stability and Composition. Presented at the 6th College Park Colloquium,
October 1981. See Origins of Life, Volume 12 (1982), pp. 245-259.
18. Organic building blocks would be destroyed even if it only took a few
minutes for them to fall from the atmosphere to the ocean. Once in the
ocean, the intense ultraviolet radiation would destroy them up to a depth of
ten meters.
19. Upon reaching the water, chemicals produced in the atmosphere would need
to combine to form DNA, RNA and proteins. To form the first cell, these
chemicals would need to be concentrated and then covered by the protective
covering called the cell wall. A serious problem for such a scenario is the
normal dilutional effect of water. Chemicals tend to disperse, causing
watery solutions to become very diluted with the progression of time. The
rate of destruction of unprotected building blocks, combined with this
dilutional effect, would greatly decrease the concentration of the "soup"
envisioned by Oparin and Haldane.
20. Denton, op. cit., pg. 261.
21. Molecular chirality results when a carbon atom is attached to four
different chemical groups or substituents. The result is molecules that are
mirror images of one another, just as our two hands are mirror images of one
another.
22. The amino acids made by Miller's experiment were among the simplest in
nature, containing only one asymmetric carbon. More complex molecules, such
as nucleotides, may contain more than one asymmetric carbon. With the
addition of each asymmetric carbon the number of possible molecules (called
isomers) doubles.
23. The terms dextrorotory and levorotary refer to the direction these
chemicals rotate the plane of polarized light. A solution of dextrorotory
amino acids rotates the plane of polarized light to the r right while
levorotary solutions to the left.
24. The Penicillin fungus makes d-amino acids to poison potential bacterial
invaders. Strychnine, an obvious poison, is also a d-amino acid and is toxic
to cellular enzymes.
25. For 80 years chemists have been trying to synthesize optically pure
mixtures of amino acids in the lab using stochastic chemical processes.
However, this has never been accomplished. According to physical chemists,
it is impossible because the two isomers have identical entropy states.
26. Miller and Urey acknowledged that the chemical makeup of their
experiment consisted of equal portions of left-handed and right-handed amino
acids.
27. Racemates are not optically active and in the laboratory always result
in proteins which contain a 50/50 mix of levo and dextro amino acids or
nucleotides.
28. In practice, laboratory experiments have shown that right-handed
building blocks have a slighter greater affinity, or attraction, for other
right-handed building blocks. Therefore, at each step in the addition of
another building block, there is a 3/7 chance that the next one added will
be the same optical isomer as the one previously added.
29. The smallest known free living life forms, bacteria, have about
12,000,000 nucleotides in their DNA. If we were to calculate the odds of
adding twelve million successive right-handed nucleotides to the growing
chain, without a single left-handed one being added, it would be 0.5 raised
to the 12 millionth power, (0.5/12,000,000)!
30. The materialist is left with a limited number of options. Either the
first life forms had a mix of right and left-handed building blocks in their
DNA and proteins, or a racemic primordial soup (which always results from
Miller-Urey type experiments), somehow defied the laws of mathematics and
gave rise to pure mixtures of left-handed amino acids and right-handed
nucleotides. The first option (racemic life) is impossible because of a
three-dimensional structure of enzymes and proper functioning is not
possible with a mix of dextro and levo amino acids. The second option is a
mathematical absurdity.
31. Some have suggested that certain clay or crystal surfaces might "select"
one isomer over another and therefore, purify a mixture of like handed
(optically pure) molecules. However, this argument ignores that fact that
the entropy states of the two isomers are identical and are very difficult
to separate. Secondly, experimental chemistry shows that it is impossible to
get pure mixtures of one or the other isomer this way. Irregularities in the
structure of the clay or crystal surfaces would result in the accumulation
of both isomers, i.e., contaminants. Since this is so, if even one incorrect
isomer gets integrated into a protein or nucleic acid, its 3-D structure
would be destroyed.
32. A minority of chemists have suggested that perhaps life started out with racemic proteins and later only the levo amino acids were selected out.
However, there is no known mechanism whereby chance chemistry can accomplish
such a "selection" process. Furthermore, the structure of proteins is so
tightly coupled to function that the intermediates between the racemic
proteins and the optically pure proteins (consisting of only levo amino
acids) would not be functional. In fact, the removal of even one amino acid
often destroys the structure and function of a protein. To change from a
racemic protein to an optically pure one would mean the substitution of 50%
of the amino acid residues.
33. Most chemicals that are poisonous to plants, animals or humans kill
their victims by binding specifically and irreversibly to the active site of
metabolic enzymes.
34. Another devastating fact regarding the "toxic waste" produced by "spark
and soup" experiments is that these chemicals, composed mainly of carboxylic
acids, bind to amino acids far more readily than amino acids bind to each
other! Therefore, it is incredulous to conclude that pure, uncontaminated
mixtures of amino acids or nucleotides could combine, or be selected out by
chance, from such a mixture of "chemical junk."
35. Anyone that has worked in a biochemistry lab knows that the smallest
amount of impurity in the reaction vessels will halt the activity and
efficiency of enzymes. In fact, the presence of common ocean water is
"dirty" enough to halt the function of free enzymes in such an experiment.
Therefore, even the ocean is an unlikely place for enzymes and life to
evolve.
36. This is called the law of mass action.
37. Recognizing the dilemma that these reversible condensation reactions
pose, some have proposed that the origin of life occurred near super hot
oceanic volcanic vents. Next to such vents the temperatures can reach
thousands of degrees, thus causing a relative decrease in the amount of
water. This would theoretically drive the condensation reaction toward the
production of "post-cursors" or proteins. The flaw in this argument is that
heat (110 degrees Fahrenheit or greater) quickly and efficiently breaks down
(denatures) proteins rendering them structurally and functionally
incompetent.
38. A solution is said to be at equilibrium when it reaches a state of
greatest entropy and lowest energy state.
39. In a solution that is in equilibrium there will always be local areas
where the dissolved particles may, for a transient period, not be evenly
distributed. That is, there will be areas of decreased entropy (increased
order). These exceptions are transient since they are in equilibrium with
the surrounding particles.
40. The Second Law, coupled with the fact that these condensation reactions
are reversible, drives the solution in the net direction of a mixture
containing predominantly unbonded building blocks. According to
thermodynamic calculations by Harold Blum (Time's Arrow and Evolution), in a
watery solution about 1% of amino acids will exist as dipeptides (two bonded
amino acids), 0.001% as tripeptides and less than one in 10/20 will exist in
a chain of ten amino acids. Those that do bond will be quickly unbonded when
a collision with water occurs unless these unlikely, reduced entropy
molecules are stored and kept away from the solution in equilibrium.
41. In
a primordial soup, random molecular movement would cause the building blocks
of life to diffuse away from their site of origin. Just as concentrated red
dye will disperse when dropped into water, the building blocks of DNA and
protein will also diffuse until equilibrium is reached. At this point there
would be billions of water molecules for every unbonded building block. This
process, along with the rapid breakdown of nucleotides and amino acids by
oxygen and UV radiation, makes it almost impossible to imagine how, in a
watery environment, biochemical precursors could combine, stay combined and
continue to build upon each other in the fact of the concept of chemical
equilibrium.
42. Enzymes are able to function as metabolic machines which extract free
energy from the environment (e.g. photosynthesis) and use this energy to
overcome the effect of equilibrium in the synthesis and preservation of DNA
and proteins. During the synthesis of proteins, enzymes first "activate" or
energize amino acids (using ATP) and allow them to bond and stay bonded.
This enormously complex process requires a specific transfer RNA molecule
for the activation and bonding of each of the twenty amino acids used in
living systems. This energy is then used to overcome the effect of
equilibrium in the preservation of DNA and proteins in a state of increased
order. In effect, enzymes capture the building blocks, bind them together,
essentially removing them from the solution, and then preserve them in their
bonded state. However, these cellular machines or mechanisms (enzymes) are
designed to store and maintain these deviations from equilibrium. The
problem for the materialist is to explain how such deviations from
equilibrium were stored in the absence of any mechanism or system capable of
doing this. Inorganic matter possesses neither the know-how nor the
mechanism to store the decreased entropy found in the chains of DNA and
proteins.
43. To overcome the effect of equilibrium, many scientists will unwittingly
assert that the addition of enough energy into a thermodynamically open
system (such as the earth) will cause the system to stray from equilibrium
and allow the accumulation of storage of "corners" of increased order (or
negative entropy). It is true that the introduction of more energy causes an
increase in the number of chemical collisions and a corresponding increase
in the number of unlikely polymers (consisting of two, three, four or more
bonded building blocks). However, in order for DNA or protein synthesis to
occur, these deviations from equilibrium must be stored or preserved. If
they (the polymers) are not stored, then collisions with water, which are
constantly occurring, will just as easily break down the randomly-formed
polymers. Furthermore, the addition of long time periods simply acts to
drive even localized "corners" of decreased entropy to a state of
equilibrium, i.e. predominantly unbonded building blocks.
44. Crystals are often presented as examples of structures that display
reduced entropy, and yet are at equilibrium. However, the order that we see
in a crystal is a secondary order which is dependent upon the order already
present in the atoms.
45. Harold F. Blum,. Time's arrow and Evolution.(2d ed., Princeton, N.J.
Princeton University Press, 1955).
46. Machines result when information (know-how) is intelligently and
deliberately combined with the natural law in matter for the purpose of
creating a purposeful mechanism.
47. For a detailed discussion on the origin of machines see The Scientific
Alternative to Neo-Darwinian Evolutionary Theory: Information Sources and
Structures. A.E. Wilder-Smith, The Word for Today Publishers, Costa Mesa,
Ca. 92628 (Phone 1-800-282-WORD).
48. A.E. Wilder-Smith, Man's Origin Man's Destiny, (1993 English ed.) The
Word for Today Publishers, (Phone 1-800-282-WORD).pp. 45-46.
49. George Wald, "The Origin of Life", Scientific American 191:48 (May
1954).
50. Blum, op. cit., 178a.
51. Blum, op. cit., 17.
52. Shapiro, op. cit., 135.
53. Harold F. Blum, Time's Arrow and Evolution (2d ed., Princeton, N.J.
Princeton University Press, 1955).
54. In Hoyle's experiment he assumed a primordial soup that contained all of
the twenty essential amino acids.
55. Nature, vol. 294:105, November 12, 1981.
56. Nature, vol. 294:105, November 12, 1981.
57. Harold Morowitz, Energy Flow in Biology (New York; Academic Press,
1968).
58. Shapiro, op. cit., 128.
59. Francis Crick, Life Itself-Its Origin and Nature, Futura, London,
(1982).
60. George Wald, "The Origin of Life", Scientific American 191:48 (May
1954).
61. See the New Testament, Hebrews 11:1.
62. George Wald, "The Origin of Life", Scientific American (May 1954).
63. Denton, op. cit., pg. 340.
64. Ibid., 341.
65. Ibid., 341.
66. Sir Fred Hoyle and Chandra Wickramasinghe, Evolution from Space: A
Theory of Cosmic Creationism (New York: Simon and Schuster, 1981), pp.
96-97.
|