|

by Erika Check Hayden
12 October 2016
from
Nature Website
Spanish version

Why many 'deadly' gene
mutations
are turning out to be harmless.
Lurking in the genes of the average person are about 54 mutations
that look as if they should sicken or even kill their bearer. But
they don't. Sonia Vallabh hoped that D178N (PrP
gene) was one such
mutation.
In 2010, Vallabh had watched her mother die from a mysterious
illness called 'fatal familial insomnia,' in which misfolded
prion
proteins cluster together and destroy the brain.
The following year, Sonia was tested and
found that she had a copy of the
prion-protein gene, PRNP, with the
same genetic glitch - D178N - that had probably caused her mother's
illness. It was a veritable death sentence: the average age of onset
is 50, and the disease progresses quickly.
But it was not a sentence that Vallabh,
then 26, was going to accept without a fight.
So she and her husband, Eric Minikel,
quit their respective careers in law and transportation consulting
to become graduate students in biology.
They aimed to learn everything they
could about fatal familial insomnia and what, if anything, might be
done to stop it. One of the most important tasks was to determine
whether or not the D178N mutation definitively caused the disease.
Few would have thought to ask such a question in years past, but
medical genetics has been going through a bit of soul-searching. The
fast pace of genomic research since the start of the twenty-first
century has packed the literature with thousands of gene mutations
associated with disease and disability.
Many such associations are solid, but
scores of mutations once suggested to be dangerous or even lethal
are turning out to be innocuous.
These sheep in wolves' clothing are
being unmasked thanks to one of the largest genetics studies ever
conducted:
the Exome Aggregation Consortium, or
ExAC.
ExAC is a simple idea. It combines sequences for the protein-coding
region of the genome -
the exome - from more than 60,000 people into
one database, allowing scientists to compare them and understand how
variable they are.
But the resource is having tremendous
impacts in biomedical research. As well as helping scientists to
toss out spurious disease-gene links, it is generating new
discoveries.
By looking more closely at the frequency
of mutations in different populations, researchers can gain insight
into what many genes do and how their protein products function.
ExAC has turned human genetics upside down, says geneticist David
Goldstein of Columbia University in New York City.
Instead of starting with a disease or
trait and working backwards to find its genetic underpinnings,
researchers can start with mutations that look like they should have
an interesting effect and investigate what might be happening in the
people who harbor them.
"This really is a new way of
working," he says.
ExAC is also providing
better information for families facing genetic diagnoses.
D178N, for example, was strongly
suspected of causing prion disease because it had been seen in
several people with the condition and seldom elsewhere. But before
ExAC, no one really had the power to see just how rare it was.
If it shows up in people more frequently
than prion disease does, that would mean Vallabh's risk of getting
the disease is much lower than predicted.
"We needed to find out if this
mutation had ever been seen in a healthy population," Minikel
says.
Data gathering
ExAC was born of frustration.
In 2012,
geneticist Daniel MacArthur was starting his first
laboratory, at Massachusetts General Hospital (MGH) in Boston.
He wanted to find genetic mutations that
caused rare muscle diseases, and needed two things:
If a mutation was more common in people
with a disorder than in healthy controls, it stood to reason that
the mutation was a likely cause.
The problem was that MacArthur couldn't
find enough sequences from unaffected people. He needed lots of exomes, and although researchers had been sequencing them by the
thousands, existing data sets weren't large enough.
No one had pulled enough together into
one combined, standardized resource.
|

Daniel
MacArthur convinced researchers
to share
genetic data on tens of thousands of people.
Sam Goresh
for Nature
|
So MacArthur started asking his
colleagues to share their data with him.
He was well suited to the task:
an early adopter of social media,
his lively blog posts and acerbic Twitter feed had made him
unusually popular and authoritative for a young scientist.
He also had a position with the
Broad
Institute in Cambridge, Massachusetts, a genome-sequencing
powerhouse.
MacArthur convinced researchers to share
data from tens of thousands of exomes with him; most were in some
way connected to the Broad.
All that remained was to analyze the
data, but that was no trivial task. Although the genes had been
sequenced, the raw data had been analyzed using different types of
software - including some that were out of date.
If one individual in the collection
showed a rare mutation, it could be real - or it could be an
artifact of how different programs 'called' the bases within,
judging whether they were,
MacArthur needed something that would
standardize this gigantic data set. The Broad had developed
genome-calling software, but it wasn't up to the task of churning
through the tremendous amount of data included in ExAC.
So MacArthur's team worked closely with
the Broad programmers to test the software and scale up its
abilities.
"That was a pretty horrific 18
months," MacArthur recalls. "We ran into every obstacle
imaginable and had nothing to show for it."
Personal stake
While this was going on, in April 2013,
Vallabh was learning how to work with stem cells at MGH while
Minikel studied bioinformatics.
Minikel met MacArthur for lunch and
explained his and Vallabh's curiosity about whether D178N existed in
healthy people. He admits to being a bit star-struck by MacArthur's
reputation.
"I thought if I could get him to
think about my problem for half an hour, that would probably be
the most important thing that happened in my whole month,"
Minikel says.
The pair went upstairs to MacArthur's
lab, where bioinformatician Monkol Lek ran a search on the ExAC data
that had been analyzed so far - about 20,000 exomes.
They didn't see Vallabh's mutation. That
wasn't good news, but, optimistic about exploring the data further,
Minikel joined MacArthur's lab.
By June 2014, MacArthur's team and its
collaborators had a data set that they were confident in - exomes
from 60,706 individuals representing various ethnic groups, who met
certain thresholds for health and consent.
They released ExAC that October at the
annual meeting of the American Society of Human Genetics (ASHG), in
San Diego, California. Immediately, researchers and physicians
recognized that the data could help to recast their understanding of
genetic risks.
Many disease-association studies,
particularly in recent years, have identified mutations as
pathogenic simply because scientists performing analyses on a group
of people with a disorder found mutations that looked like the
culprit, but didn't see them in healthy people.
But it's possible that
they weren't looking hard enough, or in the right populations.
Baseline 'healthy' genetic data has tended to come mainly from
people of European descent, which can skew results.
In August this year, MacArthur's group
published 1 its analysis of ExAC data in Nature,
revealing that many mutations thought to be harmful are probably
not.
In one analysis, the group identified
192 variants that had previously been thought to be pathogenic, but
turned out to be relatively common. The scientists reviewed papers
about these variants, looking for plausible evidence that they
actually caused disease, but could find solid evidence for only nine
of them.
Most are actually benign, according to
standards set by the American College of Medical Genetics and
Genomics (ACGM), and many have now been reclassified as such.
Similar work promises to have direct
impacts on medical practice.
In a companion paper, 2
geneticist Hugh Watkins of the University of Oxford, UK,
looked at genes associated with certain types of cardiomyopathy that
cause gradual weakening of the heart muscle.
Undetected, they can lead to sudden
death, and it has become fairly common to check relatives of people
with the conditions for genetic mutations associated with them.
Those found to have a genetic risk are
sometimes counseled to get an implanted defibrillator, which
delivers electrical shocks to the heart if it seems to be beating
abnormally.
Watkins checked the ExAC database for
information on genes that have been associated with these heart
conditions, and found that many mutations are much too common among
healthy people to be pathogenic.
About 60 genes had been implicated as
harboring pathogenic mutations that cause one form of the disease;
Watkins' analysis revealed that 40 of these probably bear no link.
This was troubling.
"If you have a genetic risk that you
believe is predicting disease but isn't, you can end up doing
drastic things that can harm someone," says Watkins.
Even some of the mutations that seem to
be reliably linked to disease aren't a sure bet - such as those in
PRNP.
There are definitely mutations in the
gene that cause the disease, but some variants might not be
pathogenic or might elevate the risk only slightly (see
'The Deadly Mutations that Weren't').
To find out the status of D178N, Vallabh
and Minikel gathered genetic data from more than 16,000 people who
had been diagnosed with prion diseases, and compared them with data
from almost 600,000 others, including the ExAC participants.
3
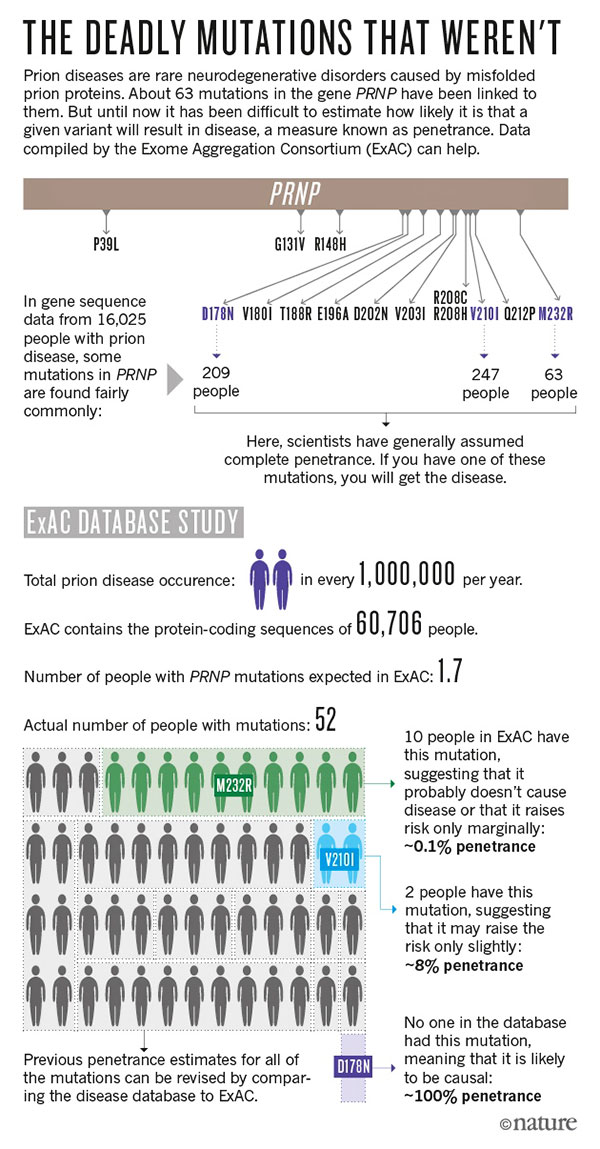
The pair found that 52 people in ExAC
had PRNP mutations that have been linked to prion diseases,
but based on the prevalence of the disease, they would have expected
to see maybe two.
Minikel calculated that some of these
supposedly lethal mutations elevated a person's risk of prion
disease slightly; some seemed not to be linked to prion disease at
all.
This work provided insight for people
such as Alice Uflacker.
In 2011, Uflacker's father, Renan, died
from
Creutzfeldt-Jakob disease, a prion illness that causes rapid
mental and physical deterioration. He was 62. Alice found out that
she carried a mutation in PRNP called V210I, which had been
linked to her father's disease in previous studies.
Three years later, she learned from
Minikel that the mutation confers, at most, a small risk of disease.
The information was helpful, and the result made sense; her
grandmother had lived to 93 despite having the same mutation.
Vallabh and Minikel would find no such
relief, however. D178N was absent from the other genomes they looked
at, and is still highly likely to cause prion disease.
Minikel and Vallabh had already begun to
suspect as much, as Minikel dug into the data.
"All along the way was gradual
confirmation of what we were assuming anyway," Minikel says.
"There wasn't any moment where we said, 'Ah, this is the worst
news.' We'd already gotten the worst news."
Human knockouts
ExAC is revealing a lot about genes
through the frequency of mutations.
MacArthur and his team found 1
3,200 genes that are almost never severely mutated in any of the
ExAC genomes - a signal that these genes are important. And yet 72%
of them have never before been linked to disease. Researchers are
eager to study whether some of these genes play unappreciated parts
in illness.
Conversely, the group has found nearly
180,000 instances of mutations so severe that they should render
their protein products completely inactive. Scientists have long
studied genes by knocking them out in animals such as mice, so that
they don't work.
By looking at the symptoms that develop,
they can study what the genes do. But that has never been possible
in humans.
Now, researchers are eager to study
these natural human knockouts to understand what they can reveal
about how diseases develop or may be cured. MacArthur and other
researchers are gearing up to prioritize which human knockout genes
to study and how best to contact the people carrying them for
further study.
But it will have to wait until he
completes the second phase of ExAC.
Due to be unveiled at the ASHG
meeting in Vancouver, Canada, this month, it will double the
data set's size to 135,000 exomes and include some 15,000
whole-genome sequences, which should allow researchers to
explore mutations in regulatory regions of the genome that are
not captured by exome sequencing.
ExAC is quietly becoming a standard
tool in medical genetics.
Clinical labs around the world now
check it before telling a patient that a particular glitch in
their genome might be making them ill. If the mutation is common
in ExAC, it's unlikely to be harmful.
Geneticist Leslie Biesecker
at the US National Human Genome Research Institute in Bethesda,
Maryland, says that his lab uses ExAC daily in patient care.
"It's a critical factor that we
take into consideration for every variant," he says.
He and other geneticists are now
embarking on a
painstaking reckoning with the genetics literature that will
probably take years.
ExAC has also driven home a point
that Goldstein and other researchers have made repeatedly:
that
failing to include people from Asian, African, Latino and other
non-European ancestries is holding back understanding of how
genes influence disease by
limiting the view of human genetic diversity.
There is now a fresh impetus to
include under-represented groups in planned studies linking
genetics and health information on large numbers of people, such
as the
US Precision Medicine Initiative.
For Vallabh and Minikel, ExAC
provided a disheartening confirmation, but also some promising
insight.
Minikel's studies have identified
3 three people in ExAC with mutations that should
silence one of the two copies of the prion protein gene. If they
can live with a limited amount of functioning protein, perhaps a
drug could be made that would silence the defective protein in
Vallabh, preventing prion aggregation and disease progression
without dangerous side effects.
Minikel got in touch with one of the
individuals, a man in Sweden, who agreed to donate some cells
for research.
Minikel and Vallabh have now joined the lab of
biochemist Stuart Schreiber at the Broad Institute, where they
are working full-time to find candidate drugs to treat prion
disease.
The couple exemplifies the challenge
of translating ExAC data into real medical benefits.
"We can't go back from this,"
Vallabh says. "We have to go through it."
Their situation couldn't be more
illustrative of what is at stake: Vallabh is now 32 - just 20
years younger than her mother was when she died.
She has no time to waste.
References
1. Lek, M. et al. Nature 536,
285-291 (2016) -
Analysis of Protein-Coding Genetic
Variation in 60,706 Humans.
2. Walsh, R. et al. Genet. Med. (2016) -
Reassessment of Mendelian Gene
Pathogenicity Using 7,855 Cardiomyopathy Cases and 60,706
Reference Samples.
3. Minikel, E. V. et al. Sci. Transl. Med. 8, 322ra9 (2016) -
Quantifying Prion Disease Penetrance Using
Large Population Control Cohorts.
|




