|

June 26, 2017
from
ScienceDaily Website
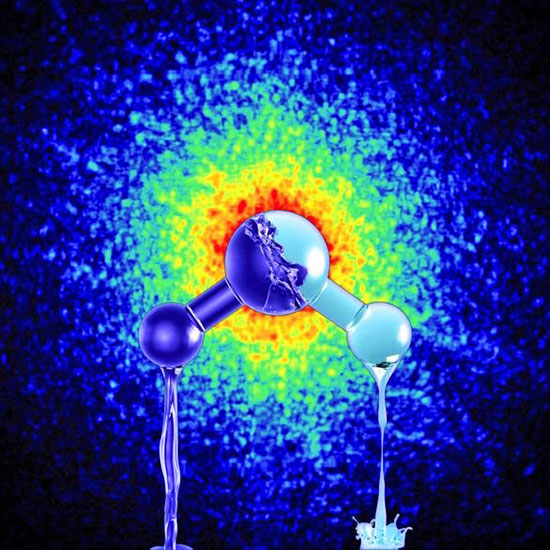
Pictured is an artist's impression of the two forms of
ultra-viscous liquid water with different density.
On the
background is depicted the x-ray speckle pattern
taken
from actual data of high-density amorphous ice,
which
is produced by pressurizing water
at very
low temperatures.
Credit: Mattias Karlén
Scientists have
discovered
two phases of
liquid water
with large
differences in structure and density.
The results are
based
on experimental
studies using X-rays.
We normally consider liquid water as disordered with the molecules
rearranging on a short time scale around some average structure.
Now, however, scientists
at Stockholm University have discovered two phases of the liquid
with large differences in structure and density. The results are
based on experimental studies using X-rays (Diffusive
Dynamics during the High-to-Low Density transition in Amorphous Ice),
which are now published in Proceedings of the National Academy of
Science.
Most of us know that water is essential for our existence on planet
Earth.
It is less well-known
that water has many strange or anomalous properties and behaves very
differently from all other liquids. Some examples are the melting
point, the density, the heat capacity, and all-in-all there are more
than 70
properties of water that differ
from most liquids.
These anomalous
properties of water are a prerequisite for life as we know it.
"The new remarkable
property is that we find that water can exist as two different
liquids at low temperatures where ice crystallization is slow,"
says Anders Nilsson, professor in Chemical Physics at Stockholm
University.
The breakthrough in the
understanding of water has been possible through a combination of
studies using X-rays at
Argonne National Laboratory near
Chicago, where the two different structures were evidenced and at
the large X-ray laboratory DESY in Hamburg where the dynamics could
be investigated and demonstrated that the two phases indeed both
were liquid phases.
Water can thus exist as
two different liquids.
"It is very exciting
to be able to use X-rays to determine the relative positions
between the molecules at different times," says Fivos Perakis,
postdoc at Stockholm University with a background in ultrafast
optical spectroscopy.
"We have in
particular been able to follow the transformation of the sample
at low temperatures between the two phases and demonstrated that
there is diffusion as is typical for liquids."
When we think of ice it
is most often as an ordered, crystalline phase that you get out of
the ice box, but the most common form of ice in our planetary system
is amorphous, that is disordered, and there are two forms of
amorphous ice with low and high density.
The two forms can
interconvert and there have been speculations that they can be
related to low- and high-density forms of liquid water.
To experimentally
investigate this hypothesis has been a great challenge that the
Stockholm group has now overcome.
"I have studied
amorphous ices for a long time with the goal to determine
whether they can be considered a glassy state representing a
frozen liquid," says Katrin Amann-Winkel, researcher in Chemical
Physics at Stockholm University.
"It is a dream come
true to follow in such detail how a glassy state of water
transforms into a viscous liquid which almost immediately
transforms to a different, even more viscous, liquid of much
lower density."
"The possibility to make new discoveries in water is totally
fascinating and a great inspiration for my further studies,"
says Daniel Mariedahl, PhD student in Chemical Physics at
Stockholm University.
"It is particularly
exciting that the new information has been provided by X-rays
since the pioneer of X-ray radiation, Wilhelm Röntgen, himself
speculated that water can exist in two different forms and that
the interplay between them could give rise to its strange
properties."
"The new results give very strong support to a picture where
water at room temperature can't decide in which of the two forms
it should be, high or low density, which results in local
fluctuations between the two," says Lars G.M. Pettersson,
professor in Theoretical Chemical Physics at Stockholm
University.
"In a nutshell: Water
is not a complicated liquid, but two simple liquids with a
complicated relationship."
These new results not
only create an overall understanding of water at different
temperatures and pressures, but also how water is affected by salts
and biomolecules important for life.
In addition, the
increased understanding of water can lead to new insights on how to
purify and desalinate water in the future.
This will be one of the
main challenges to humanity in view of the global
climate change.
Story Source
Journal
Reference
-
Fivos Perakis,
Katrin Amann-Winkel, Felix Lehmkühler, Michael Sprung,
Daniel Mariedahl, Jonas A. Sellberg, Harshad Pathak,
Alexander Späh, Filippo Cavalca, Daniel Schlesinger,
Alessandro Ricci, Avni Jain, Bernhard Massani, Flora Aubree,
Chris J. Benmore, Thomas Loerting, Gerhard Grübel, Lars G.
M. Pettersson, and Anders Nilsson -
Diffusive Dynamics during the
High-to-Low Density transition in Amorphous Ice
- PNAS, June 26, 2017 DOI: 10.1073/pnas.1705303114
***
H2Oh! Water is actually Two Liquids
Disguised as One
by
Katharine Sanderson
30
May 2018
from
NewScientist Website

Earth's most
precious liquid is weird,
and if it wasn't we would
die.
Now experiments have
uncovered its secret:
it's not one liquid, it's
two
"WATER is very
strange," says Anders Nilsson.
He should know: he
has been studying the stuff for most of his working life.
His claim may be hard
for the rest of us to swallow - after all, what could be more
ordinary than water? Its behavior is so familiar, its appearance
so commonplace, that we are tricked into assuming that it is
more or less the same as everything else. But water is uniquely
weird.
If it weren't, none
of us would be here to notice.
For example, if water weren't densest at around 4°C rather than
as ice, lakes and rivers would freeze from the bottom up, slowly
killing their inhabitants.
If it weren't so
spectacularly good at absorbing heat, the planet would have
boiled over long ago. And if its molecules, barreling through
membranes or darting down veins, weren't so good at sweeping
other chemicals along, plants and animals would die of
malnutrition.
Scientists have been plumbing the depths of water's strangeness
since at least the time of Galileo, to no avail.
But now, thanks to
the work of Anders Nilsson and others (Diffusive
Dynamics during the High-to-Low Density transition in Amorphous
Ice), we might be on the verge of
understanding why it behaves the way it does.
Their explanation is
as strange and wonderful as the stuff itself:
water isn't one
liquid, but two...
| 


