|
produced by Craig Venter and his colleagues SCIENCE PHOTO LIBRARY
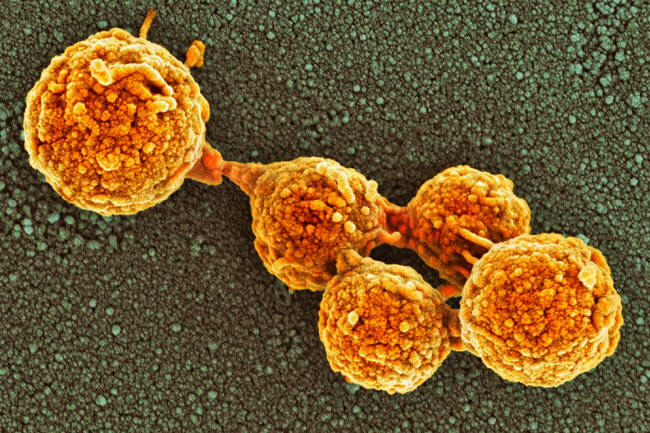
Synthetic cells made by combining components of Mycoplasma bacteria with a chemically synthesized genome can grow and divide into cells of uniform shape and size, just like most natural bacterial cells.
In 2016, researchers led by Craig Venter at the J. Craig Venter Institute in San Diego, California, announced that they had created synthetic "minimal" cells.
The genome in each cell contained just 473 key genes thought to be essential for life.
The cells were named JCVI-syn3.0 after the institute and they were able to grow and divide on agar to produce clusters of cells called colonies.
But on closer inspection of the dividing cells at the time, Venter and his colleagues noticed that they weren't splitting uniformly and evenly to produce identical daughter cells as most natural bacteria do.
Instead, they were producing daughter cells of bizarre shapes and sizes.
But their definition of what was necessary for growth turned out to be what was needed to make beautiful colonies growing on an agar plate, she says, rather than what was needed to produce cells that divide in a uniform and lifelike way.
By reintroducing various genes into these synthetic bacterial cells and then monitoring how the additions affected cell growth under a microscope, Elizabeth Strychalski and her team were able to pinpoint seven additional genes required to make the cells divide uniformly.
When the researchers added these seven genes to JCVI-syn3.0 to produce a new synthetic cell, they found that this was enough to restore normal, uniform cell division and growth.
Strychalski and her colleagues found that while two of the seven genes were already known to be involved in cell division, five were previously without a known function.
This is because the minimal cell is a good analogue of the last universal common ancestor of all life on Earth.
The new finding also,
Journal reference
|