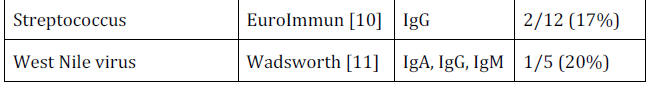|

by
David Crowe
May 13, 2020
from
TheInfectiousMyth Website
Assuming that a new virus called COVID-19 was actually
discovered, we are being told that antibody tests are a
vital tool for determining who is immune and who is not.
These tests are heralded as 'essential' and 'necessary,'
despite some downplayed doubt among "experts" about how
reliable they are.
Canadian author and long-time independent researcher,
David Crowe, has written a new paper, "Antibody Testing
for COVID-19."
I can safely say it is the most detailed analysis of the
tests anyone will ever read.
Source
It is now time for a
discussion of antibody testing.
Many people now want to
know how many have been silently infected in the general population,
how many are immune, and how this affects the fatality rate.
This requires antibody
testing and there is at least as much interest in this now, as there
has been in the COVID-19 RT- PCR RNA testing that is used to declare
someone infected.
Executive
Summary
A positive RT-PCR test is used to tell people that they have
COVID-19 RNA and are deemed infected and infectious, despite the
technology's numerous flaws and known false positives.
Antibody
tests are now being used under the assumption that someone who is
positive for antibodies for COVID-19 has previously been infected
and, if they have recovered from symptoms, is now immune.
Antibodies are our body's immune system reaction to viral proteins,
known as antigens. Antibody tests incorporate antigens, and a
chemical that allows the intensity of the reaction to be measured
using light.
Ideally antigens would come from pure virus, but
COVID-19 virus has never been purified, thus antigens are created
artificially from proteins based on portions of the 30,000 base RNA
genome that is believed to come from the virus.
The major antibody types that are looked for are IgM, believed to be
a generic infection fighting antibody that arises about a week or so
after infection, and IgG, believed to be more specific, and believed
by some to take longer for the body to create.
After the infection
is resolved, IgM antibodies are believed to gradually disappear,
while IgG remain, providing ongoing immunity.
Unfortunately, this idealized picture is not supported by the
available evidence, either because the evidence does not exist, is
insufficient, or because it directly contradicts the model.
Positive antibody tests should be impossible before the person is
first infected (RNA positive). Yet, old blood samples (2019 or
before) have tested positive in significant numbers.
Almost 14% of
saved blood from old donations tested positive in a Dutch study, and
in the validation of the Cellex and Chembio tests, 4.4% and 3.6% of
old samples were positive.
The idealized antibody model is based on the date of infection as
the starting point, but this date is never known with certainty.
Even when someone came into contact with a COVID-19 RNA positive
person on a certain date that is not a guarantee that this was the
date of infection, given that, prior to the lockdown, people could
apparently be infected while playing in the park, eating at a
restaurant, walking
down the street, attending a concert, or participating in any other
now banned activity.
When antibody surveys are performed, the vast
majority of people who test positive had no idea that they had
previously been infected, and cannot possibly be sure about the
date.
Thus, the incubation period for the virus is impossible to
determine accurately, as well as the range of days after infection
that IgM and IgG start to develop.
This makes an accurate antibody
model impossible to construct based on currently available data,
despite numerous beautiful graphs showing this model in idealized
form.
Simple models that illustrate the timing of antibodies show the
quantity (titer) rising smoothly and, for IgM, eventually peaking
and declining smoothly. Yet many studies have found negative tests
throughout the symptomatic period.
A test developed by the Wadsworth
Centre in New York found 40% of samples negative for antibodies
11-15 days after symptoms started, and even more between 16-20 days.
This indicates that antibodies may come and go randomly and not
behave in a smooth and predictable fashion.
No test documentation, antibody surveys or scientific studies showed
the disappearance of IgM antibodies, predicted by the model, perhaps
because it does not happen, or it takes more than 30 days, the
maximum examined.
This might not be terribly important in practice,
but it is another indication that the beautiful models shown in the
form of graphs are simplistic, if not outright wrong.
Other problems with antibody tests include a significant number of
samples testing antibody positive from people who were COVID-19 RNA
negative (although some had 'COVID-like' symptoms), with no evidence
that the person was ever infected. In one Chinese study the positive
rate on presumably never infected people was 25%.
Antibody tests, like most infectious disease tests, are often
reported as 'Positive' or 'Negative', but the results are really
whether the intensity of a color change in the test kit was above or
below an arbitrary number.
The reliability of this was called into
question, inadvertently, by one test manufacturer, who showed that
continually diluting samples 50:50 did not result in a halving of
the color change at each step. In some cases, less material resulted
in significantly more intense color changes.
Researchers have tried to connect the antibody titer (in reality,
this is just the color change intensity) with the severity of
symptoms, but two Chinese papers that studied this had to admit that
there was no difference between mildly and severely symptomatic
people in the quantity of antibodies, nor between those with or
without pre-existing conditions, nor in the duration of symptoms.
Test manufacturers always run their test on blood samples from
people with unrelated medical conditions as a check.
Even though
only a small number of samples were examined, for a small number of
conditions, different manufacturers found a significant percentage
of samples positive for COVID-19 antibodies, that were known not to
have COVID-19, but instead contained other viruses, bacteria or mycoplasma, or were from people with auto-immune conditions,
indicating that the antibodies are not specific.
For example, 10% of
Hepatitis B samples were positive,
33% of Respiratory Synctitia Virus, 10% of auto-antibodies and 17%
of Streptococcus.
A large number of population surveys have been compiled by
Dean
Beeler and they reveal a wide range of percentages of populations
antibody positive, from less than 1% in many cases to 32% in a poor
part of Boston.
This is generally seen as an indication of how far
through the population that the virus has rampaged. One flaw of most
of these surveys is that the population is chosen non-randomly, and
does not represent the general population.
The group may be a
household survey, volunteers, high school students and staff, health
care workers, blood donors, or people going for blood tests at a
lab.
But a far bigger problem is that the number produced is impossible
to validate. When 1.5% of Santa Clara volunteers tested positive, it
was assumed that that was truth. This 'truth' asserts that all of
these people were RNA-positive at some point in the recent past.
But
there is absolutely no evidence for this.
The 'truth' assumes that
all the people were negative for COVID-19 antibodies prior to the
assumed period of RNA-positivity.
But there is absolutely no
evidence for this.
It assumes that the 98.5% who tested negative were never
RNA-positive.
But there is absolutely no evidence for this.
It
assumes that the 98.5% never had the antibodies being looked for
before.
But there is absolutely no evidence for this.
I could assert that the real fraction positive in Santa Clara was
98.5%, not 1.5%, and there is no less evidence for my assertion than
for the results from antibody testing.
These surveys often ask if people who tested antibody positive had
'COVID-like' symptoms in the last few weeks or months (and most say
that they did not).
But these symptoms (fever, cough, loss of smell
or taste, fatigue) are so generic that they are absolutely not
evidence that the people were previously COVID-19 RNA positive.
One solution would be a time series survey of a large number of
people currently negative on both RNA and antibody tests (uninfected
and never infected). Every few days these people would give a drop
of blood and a nasal swab.
Some would become RNA positive, and then
could be examined more frequently for the exact pattern of antibody
development, through to the disappearance of IgM antibodies.
This
experiment would be time consuming, intrusive, inefficient (as most
people may never become infected) and expensive.
But considering the
vast sums of money spent on COVID-19 research, quarantining and
treatment, and the even more tremendous sums of money lost by a
hobbled economy, and the assertion of our politicians that they
follow the science (not the head lemming), this would surely be
worthwhile.
Antibody tests might be fatally flawed, but they can be used in
highly destructive ways. If the number of people who are antibody
positive remains below the level of 'herd immunity' (90% or so) it
will be an excuse to promote or even mandate vaccination, after a
vaccine is rushed onto the market.
Antibody tests could also be used
to indefinitely quarantine people who do not test positive,
asserting that they are at danger of becoming infected, and then
spreading it to others.
They could be,
used to separate families, arguing that the children must be put in
foster homes because the parents are at risk of an infection at any
time.
Faulty tests have been used to indefinitely quarantine Chinese
citizens.
But now, do we have more civil rights in the UK, United
States, Canada or other modern, once democratic countries?
We have been here before. A BBC story from 2008, "Life Sentence",
always makes me cry. Starting in 1907 nearly 50 women were locked in
an asylum within the Long Grove insane asylum in Surrey because they
were deemed carriers of typhoid.
They were sane and healthy when
they entered, but most were driven mad by the solitary confinement,
by humiliations like toilets that flushed boiling water, warmly
reminding them that even their excrement was a danger to the world,
by the nurses wearing PPE.
After they stopped imprisoning such women
in the 1950s, the prisoners remained. In 1992, when the asylum
closed for good, the three remaining women were deemed insane and
relocated to other institutions, their entire lives destroyed by an
infectious panic.
Despite this, the UK Department of Health told the
BBC that there never had been a policy of incarcerating people
deemed carriers of an infectious disease. [32]
This document is based on an examination of all antibody test
documentation submitted to the US FDA (Food and Drug Administration)
and a series of antibody surveys of groups of people from around the
world.
A Little Background
COVID-19 is alleged to be an RNA virus, so the RNA will be in your
body as soon as you are infected.
RT-PCR is an ultra-sensitive test
(capable of reliably detecting as few as five molecules of RNA in a
sample, and possibly triggering on just one) and therefore should be
positive almost immediately after infection. 1
1
Often only samples from some areas of the body are positive (e.g.
nose but not throat or stool), leading to the belief that the virus,
unlike blood borne viruses, only colonizes a small part of the
respiratory tract. Samples from deep in the nose (nasopharyngeal)
are believed to be most reliable for early detection. [27]
IgM antibodies are believed to be produced by the body as generic
infection fighters, soon after infection. An infected person will
not be IgM positive immediately, but within a few days at most.
These antibodies persist for a while after the infection is
resolved, but then fade away.
IgG antibodies are believed to be produced by the body as very
specific fighters of a particular invader, such as COVID-19.
Some
scientists believer they take longer than IgM to be produced, but
all agree that they persist long after the infection is resolved,
possibly for a lifetime.
Antibodies and Antigens
Antibodies are believed to be generated by the immune system in
response to a foreign protein, known as an antigen. In the case of
COVID-19, an antigen would be a protein probably found on the outer
shell of the virus (because the internal proteins
are unlikely to stimulate an immune reaction).
When an antibody
binds to an antigen, it is a signal to the body to destroy the
foreign object, such as a virus particle.
Antibody tests contain one of more of these antigens, that are bound
to chemicals that produce some kind of color change or fluorescence
when an antibody binds to them. The result of the antibody test is
read as the intensity of this color change or fluorescence.
This
makes reading tests results easier to automate.
The antibody-antigen reaction is continuous, and not binary, not
naturally 'negative' or 'positive'. Therefore, manufacturers
recommend a particular intensity of color change or fluorescence as
the division between 'negative' (antibodies not present) and 'positive' (antibodies present).
Some manufacturers recommend an
intermediate zone between negative and positive, and specimens in
this zone may be re-tested, possibly immediately, or possibly in the
future, when it is believed that, if the reaction is real, antibody
levels will have increased to a clearly detectable level.
Since antigens are viral proteins the obvious place to obtain them
would be from purified virus. However, since COVID-19 virus has
never been purified, this is currently impossible.
In lieu of this, traditional, impure materials (e.g. nasal swab)
would be added to a cell culture, and proteins that were believed to
be viral would be purified and used as antigens.
But in modern tests
most antigen proteins are 'recombinant', produced artificially from
the published 30,000 base RNA sequence believed to be COVID-19.
Sources of Data
This article is based on a review of all antibody tests approved
under FDA Emergency Use Authorization, [33] a list of surveys
maintained by a third party [23] and several medical papers.
Status of Antibody Tests
The only jurisdiction with a formal structure for approval of
antibody tests is the United States but, until very recently, it was
just a charade, as the test manufacturers did not need to provide
validation data. Now, validation data must be provided, but the FDA
can only do a paper analysis. [3]
Imagine if auto-manufacturers had to build cars to certain EPA (US
Environmental Protection Agency) fuel efficiency standards, but
rather than sending a car to the EPA for testing, they could do the
testing at their facilities, and just send the results in
afterwards.
Then, there would have been no need to write software to
fake the fuel efficiency by running the engine differently under
testing conditions.
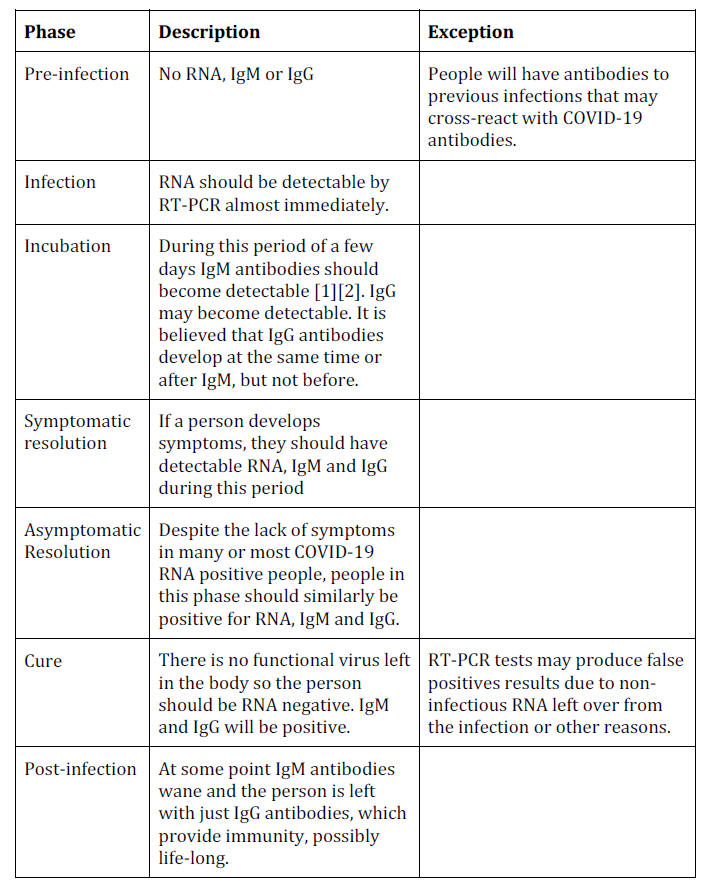
A Theoretical Timeline
The theoretical timeline of an RNA virus disease is shown below:
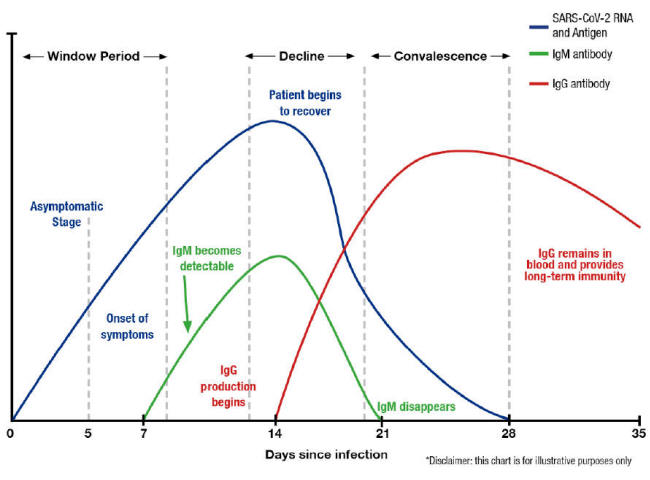
A graph from the test manufacturer Diazyme illustrates this belief,
which indicates that the immune system is aware of the concept of a
7-day week (other similar graphs indicate that, for other viruses,
multiples of 10 days are preferred). [26]
A paper from the Journal of the American Medical Association differs
in showing IgM and IgG antibodies arising at the same time:
[27]
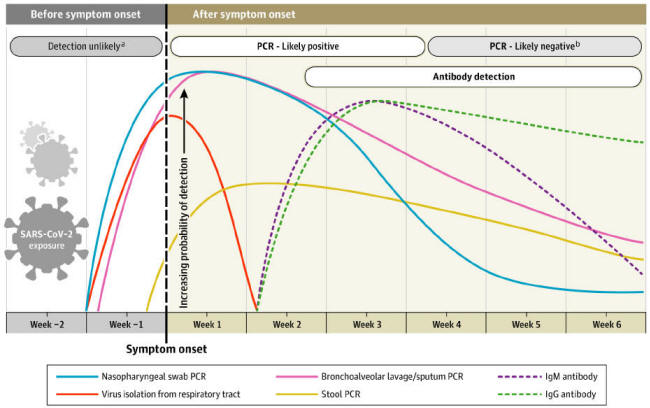
This paper will show that COVID-19 antibody testing does not support
this timeline.
Timeline in Practice
Pre-Infection: No Positive Test Results
Before people are infected with COVID-19 they should theoretically
be negative for RNA and all types of antibodies.
In the following table, note that tests for only one antibody type
will perform better as they only have one chance for a false
positive, whereas tests for multiple antibody types could test
positive for any type.
The lowest value is shaded in blue, and the
highest in red.
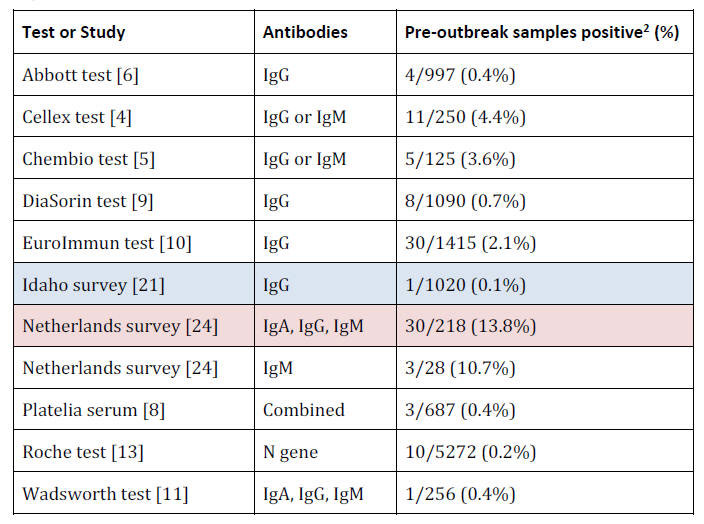
Infection: RNA-Positive Only
Theoretically, someone who has just been infected with COVID-19 will
be rapidly positive for RNA (due to the sensitivity of the test) but
it will take a few days for antibodies to develop.
There is no data
available at present, as it would require daily blood samples from a
large number of people who were initially negative for all tests, so
that the time series could be examined.
This type of testing could
validate all aspects of the theoretical timeline, but would also be
very expensive, intrusive (daily swabs and blood tests) and would
need a very large number of people
because most may never have any positive tests and, ahead of time,
it would be impossible to tell who would eventually become
RNA-positive.
2
Some tests had a 'borderline' or 'indeterminate' category and these
were counted as positive.
The best that can be done is to guess at the date of infection based
on contact with someone who later tested RNA positive, but there is
never proof that this was actually the date of infection, it is
still just a supposition.
The time at which someone first develops symptoms or learns they are
RNA- positive is not very useful because it may occur a variable
number of days after they are infected.
Incubation: Antibodies Start to Develop
This part of the theoretical timeline has the same problem as the
moment of infection and would also require time series with daily
testing of a large number of people.
But perhaps we can sometimes be lucky and someone will be tested
early enough that they will be RNA positive and the development of
first IgM and then IgG antibodies.
In the Chembio test validation IgG antibodies were found in all four
RNA positive samples collected within 6 days of the development of
symptoms, but IgM antibodies only in one out of four [5]. It should
have been the other way round if IgM occurs before IgG.
A study of 30 severely and mildly ill COVID-19 patients found that,
"a higher proportion of patients…had earlier IgG than IgM
seroconversion [first detection of antibodies]". [28]
Some tests and studies made it impossible to validate this theory
because they used total antibodies, not distinguishing between IgM
and IgG (Platelia). [8]
Many other tests only reacted to IgG
antibodies, so comparison with IgM was not possible.
There is limited information but it does not support the notion held
by some that IgM antibodies develop before IgG.
This is consistent with the first SARS coronavirus in which IgG
antibodies were found before IgM antibodies, calling into question
the usefulness of IgM antibodies as an early warning system.
[25]
And, given that IgM antibodies disappear over time, they are not
useful for determining later immunity either.
Symptomatic Resolution: RNA and Antibodies
Once symptoms are noticed, enough time should have passed for IgM
antibodies to develop, so during the days or weeks of resolution of
symptoms every patient should be positive for RNA, IgM and IgG.
During SARS, also blamed on a coronavirus, a small sample of
isolated patients mostly developed IgG antibodies by 14 days after
symptoms, and all by 30 days. [25]
The Chembio test found IgG antibodies in 100% of RNA-positive
samples from 0-21 days after first symptoms, except for 4/10 (40%)
of samples collected between 7-10
days.
The EuroImmun test had positive IgG results sporadically from
the first day of symptoms through day 15, and then consistently
through day 36, the last day tested, while negative tests were found
from the day of symptoms through day 18.
There were very small
numbers of tests performed on each day (1-6) with an average of less
than two tests per patient. [10]
The Abbott IgG test had 0 positive results within 3 days of first
symptoms, 25% positive within 3-7 days, 86% within 8-13 days and
100% after 14 days. [6]
Similarly, Diasorin found 11/44 (25%)
positive for IgG within 5 days of first symptoms, 44/49 (90%)
between 6 and 14 days, and 40/41 (98) after 15 days. [9]
The Ortho Vitrios test found 8% IgG negative to the 'N' gene within
5 days of the person testing RNA positive, but the fraction then
went up, 11% on tests 6-15 days after RNA positivity, and 25% during
the 16-22 day period. [12]
They also tested people a known number of
days after symptom, and again a significant fraction were negative:
8% 12-17 days after symptoms and more, 17%, 18-32 days after
symptoms.
The Wadsworth test [11] simultaneously detects IgA, IgG and IgM
antibodies, so cannot be used to distinguish the timing of different
antibodies.
However, it surprisingly
had negative results on 40% of samples from people who were known to
have been,
-
RNA positive for
11-15 days
-
43% positive for 16-20 days
-
12% positive for more
than 20 days
If indeterminate results are included with negative
(since they are not clearly positive) the percentages are,
-
69% (11-15
days)
-
51% (16-20 days)
-
21% (over 20 days)
Two additional
studies with the Wadsworth test showed that, at least 25 days after
symptom offset, 6% were antibody negative, and 12% were either
antibody negative or indeterminate.
In other words, negative results
for IgA, IgG and IgM were found long after some antibodies should
have developed.
Different tests give very different results, from Chembio, positive
for IgG on all days after symptoms developed, to Abbott which had
only 25% positive within 3 days of symptoms developing.
This
indicates that the tests are not all measuring the same thing, or
not with the same level of sensitivity. Additionally, the relevant
timing is from the date of infection, not symptoms, and that is
unknown in almost every case.
A survey of 85 COVID-19 patients in Wuhan, China found that the
majority of samples had detectable IgM antibodies from the first day
measured to 30 days or beyond, but there was no time where all tests
taken were positive (the maximum was 94% on day 19 after symptoms).
IgG samples taken on day 30 or later were 100% positive, but only 14
out of 85 patients were tested during this period. Prior to 30 days
all groups of samples had at least 9% negative, and some as much as
60%.
A large flaw in all the validations is that the people sampled at
different times are not the same, so individual anomalies (such as
the disappearance of IgG antibodies, and then reappearance) cannot
be seen.
Again, a time series could provide information that shows
that the development of antibodies follows a predictable pattern in
individuals.
The only thing approaching a timeline is found in the Abbott test
documentation which shows two people who had two negative IgG tests
followed by several positive tests.
The two people, however, seroconverted at rather different times.
One between days 6 and 7
after symptoms and the other between 10 and 11. [6]
As usual, the
amount of time from infection to the development of antibodies was
unknown. Since the Abbott test is IgG only, there was no information
on IgM.
In summary, for much of the test documentation, there were a mixture
of positive and negative IgG and IgM test results over much of the
time tested, and for some tests, right up to the end of the period.
This could be due to large variations in the development of
antibodies in each person, false results from certain test kits, or
both.
Asymptomatic Resolution: RNA and Antibody Positive
From a testing perspective the asymptomatic resolution of an
infection should also be a time when people are positive for RNA,
IgM and IgG.
The problem is that the person affected is not sick,
and much less likely to be tested.
Again, a time series of many
people could identify asymptomatic infections, and could test the
hypothesis that these people would first become RNA positive, then IgM positive, then IgG positive before the resolution of the
infection.
The information that is available on the appearance of antibodies in
asymptomatic people who are RNA-positive is absent the date of
infection, which is the only date that matters.
Therefore, there is
no useful information on this theoretical phase.
Post-Infection: Disappearance of IgM Antibodies
IgM antibodies should disappear after a person has eliminated the
virus (becoming RNA negative).
The Chembio test validation obtained samples from 2 people at 21
days after symptoms, and both were IgM positive. [5] Similarly, a
survey of 85 patients in Wuhan, China, followed patients for over 30
days and did not document the disappearance of IgM. [29]
Additionally, the disappearance of IgM antibodies implies that they
appeared in the first case, but even in people who are RNA positive
with symptoms, there are still sometimes negative IgM tests.
This is
often masked by considering someone who is IgM OR IgG positive, to
be antibody positive.
The documentation available does not exclude
the possibility that some people never generated IgM antibodies.
The data provided in this article does not support the notion that
IgM antibodies eventually disappear, but it may just be because the
patients were not followed long enough.
Performance Issues
Positive Results on Coronavirus Negative People
COVID-19 antibody tests should only very rarely be positive on
people who tested RNA negative (who were likely tested multiple
times, using samples from different areas of the body), even if they
were hospitalized for symptoms that might have seemed 'COVID-like'
but actually tested RNA negative.
There is always the possibility
that some of these people previously had a COVID-19 infection,
probably asymptomatic (otherwise they would likely have been
tested), but in none of these cases was there any evidence for this.
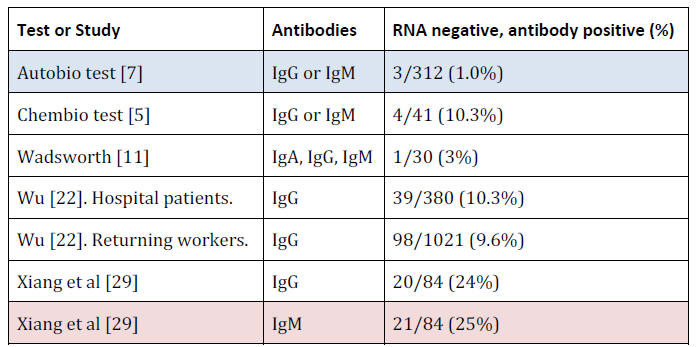
Antibody Measurement Performance
Antibodies are generally measured by a color change which can be
monitored by reflectance, fluorescence or optical density.
The color
change should deepen, or the fluorescent glow should increase, with
the quantity of virus in a predictable (preferably linear) fashion.
In other words, if the blood is diluted 50% then the reflectance,
fluorescence or optical density should drop by half.
In the Chembio validation, when blood samples were continuously
diluted by half, they did not follow a pattern of optical
reflectance that was related to the amount of dilution.
With one
sample, after reflectance dropped from 36 to 16 on the first
dilution (close to half, as expected) the reflectance stayed between
11 and 16 until the fifth dilution where it rose to 24, which was
almost considered a positive result (25 was the cutoff).
On the
second sample, the IgM reflectance almost doubled on the first
dilution (as opposed to dropping). This was the only test validation
that included a similar experiment, so there is no evidence that
antibody testing results can be used to estimate the quantity of
virus.
It also calls into question the meaningfulness of a numeric
cutoff in the first place, to distinguish positive from negative
(and possibly borderline or indeterminate).
Disease Severity Predictive Value
The amount of antibody, measured by surrogates like reflectance or
optical density, is often measured, with the implication that the
level of antibodies reflects the severity of the disease.
One survey
of COVID-19 patients examined two types of IgM and IgG levels
(anti-NP [internal nucleoprotein] and anti-RBD [surface spike
protein receptor binding domain]) for a group of 7 severely ill
patients and a group of mild case and concluded that,
"Serum
antibody levels were not correlated with disease severity".
[28]
There was similarly no obvious pattern in the same study with
patients with or without co-morbidities.
A paper from Shanghai studied antibody titers (levels) in 175
recovering COVID-19 patients, and found a weak correlation with age,
but no correlation with people who never developed high levels of
antibodies and the duration of disease. [31]
Cross Reactions
Antibody tests are often subject to cross-reactions with other
conditions.
This could be because the medical condition produces
similar antibodies, or because something related to that condition
reacts with other test components.
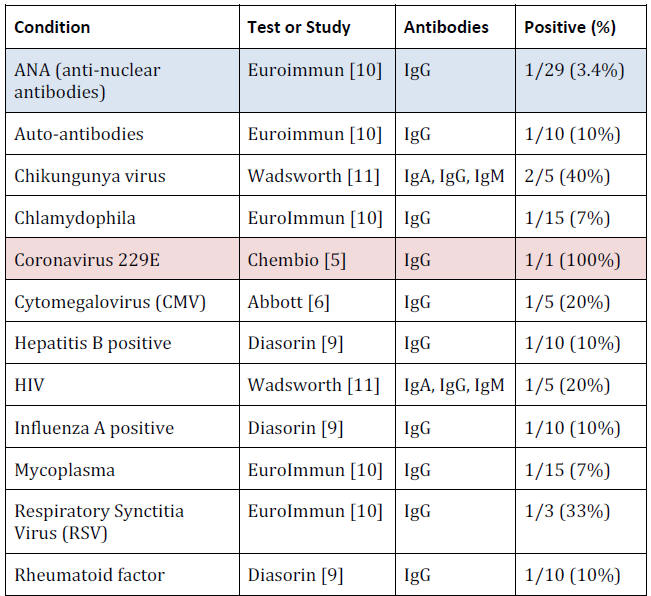
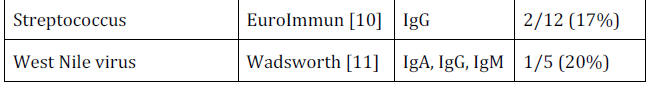
The choice of conditions to check for is completely under the
control of the manufacturer and even when no cross reactions were
found for a condition, the number of samples tested was so small
that the possibility of a fairly high rate of false positive cross
reactions still exists.
For example, a sample of 10 cannot show that
even a 10% false positive rate is unlikely.
General Criticisms of Tests
Even where test validation data conforms to the expectation about
the behavior of antibodies, there are criticisms that can be made:
-
Manufacturers are responsible for providing the data, and they know
there is no point in submitting data with major red flags, meaning
that they can spend time adjusting the samples they are using, and
how they are analyzed to ensure that the submitted report makes
their test looks good.
-
There is no way to validate the manufacturer validation data.
-
There is no consistent set of validation tests that need to be
performed by all manufacturers.
-
Time series from the time of infection through at least the decline
of IgM antibodies are not provided in any case.
-
When information is provided over time, it is not for the same
people.
-
Timing of antibody results is from the day of first symptoms, or the
day of testing RNA-positive, not from the earlier date of infection.
-
In many validation tests only tiny numbers of samples are tested.
Sometimes a cross-reaction was searched for by testing only one
sample. Yet, with even 1% cross reactions being important, well over
100 samples would be needed.
-
Only a limited number of conditions were searched for
cross-reactions.
-
Since the tests were validated by the manufacturers in ideal
environments, it can be predicted that performance will be lower
when used in practice by purchasers of the tests.
These flaws in antibody tests are fatal.
At present no antibody
tests are properly validated, and the results cannot be relied upon,
particularly not to make sweeping changes in society, such as
mandatory vaccination and quarantine of people who do not have the
'right' antibody test results.
Population Surveys
Several surveys of local populations for antibodies have been
undertaken.
In many cases this is to estimate the penetration of
COVID-19 into the general population, who have mostly been
asymptomatic, or experienced only minor symptoms.
Population being Surveyed
It is very hard to compare these surveys because they use completely
different samples of people.
Some are random household surveys,
although randomization may be reduced by allowing multiple household
residents to participate. Others are surveys of blood donors, people
who have given blood at a lab for reasons unrelated to COVID-19,
volunteers recruited by Facebook ads, or at a testing center in a
public place.
No survey can be taken as representative of the
general population.
Validating the Fraction Positive
The result of a population survey that everyone is interested in is
the percentage positive.
This is generally much higher than expected
by those who focus on the number of known cases, by dramatically
expanding the number of likely cases.
These surveys lead to the
conclusion that the death rate from COVID-19 is greatly exaggerated
(especially in two California surveys) and that herd immunity may
occur naturally.
But there is no evidence that the fractions of the population that
are antibody positive are meaningful, for several reasons:
-
The presence of antibodies is taken to mean that the person was
previously RNA positive with no symptoms, or minor symptoms. None of
the surveys have proof that all the people, or even a majority, were
previously RNA- positive (and presumed infected), and the time has
obviously passed to obtain this information.
-
The people were assumed to be antibody negative prior to becoming
RNA positive. None of the surveys have evidence for this.
-
The absence of antibodies is taken to mean that the person was never
COVID-19 RNA positive. None of the surveys have evidence for this.
-
It is assumed that the tests used would all give approximately the
same result. Since there has been no cross-validation of tests, this
is an unfounded assumption.
Virus purification cannot
be used to validate antibody tests when the virus is believed to
have been defeated and is no longer in the body.
Only a time series could
identify people who become RNA-positive, and then monitor their
antibody development over time.
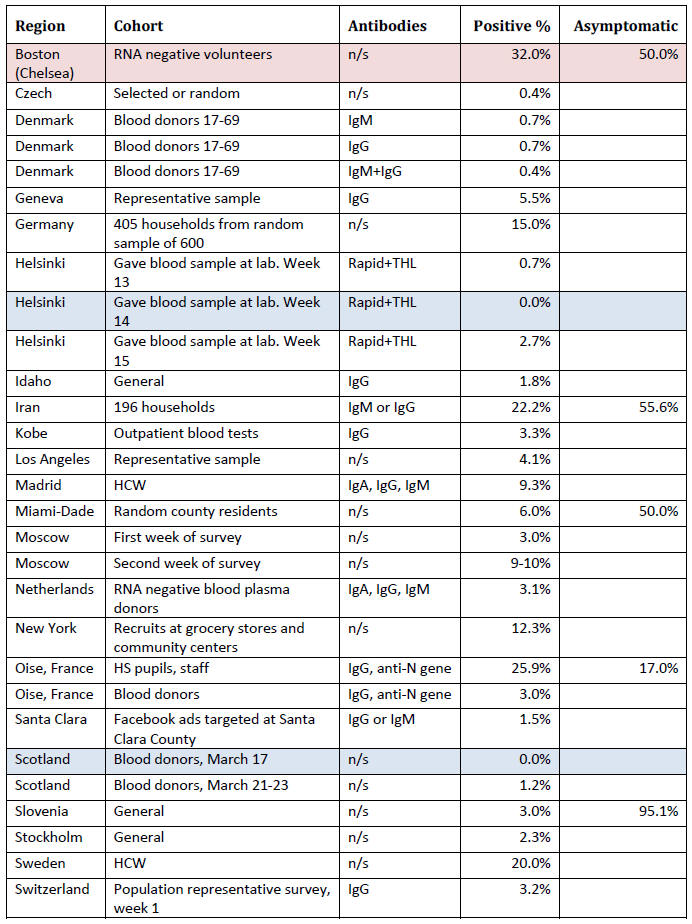
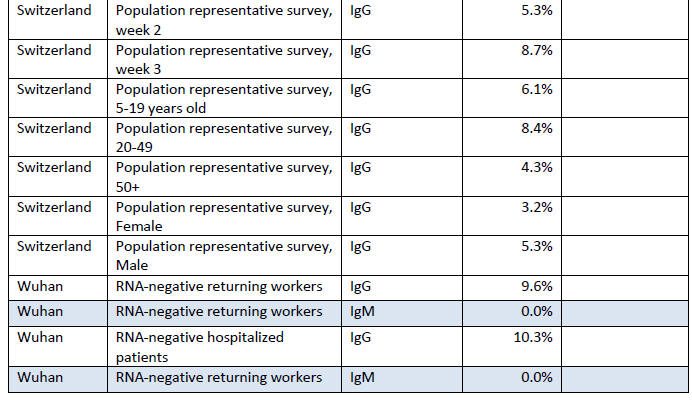
Summary of Fraction Positive
This section contains information from antibody surveys in a table
maintained by Dean Bealer. [23]
It shows the group being surveyed
(cohort), the type of antibodies
looked for (not always provided), the percentage who were antibody
positive and, in some cases, the percentage who were asymptomatic in
the weeks before the test.
Acronyms used in the table:
At present this information is simply provided as a convenient
summary.
Drawing conclusions from it is difficult, except to say
that if the antibody tests can be believed, in no area have the
majority of people been infected.
On the other hand, the results may
not even be close to the number of people who actually did
experience an infection as there is no way to validate an antibody
test in the general population, without historical records of coronavirus
'infection' status (i.e. a time series documenting RT-PCR RNA positivity and the subsequent development of antibodies).
Where the fraction of people who had been asymptomatic in the weeks
before the antibody test (not on the date of the test, as the
infection has presumably been resolved some time ago), among a group
of antibody positive people, was reported, the numbers were all over
half, except for the study in Oise, France, in which participants
were asked to report any respiratory symptoms over the last three
months, which were mostly runny nose, cough, headache, tiredness,
sore throat and fever.
About half were listed as having "major"
symptoms (so half were asymptomatic or had minor symptoms), but 'major' symptoms included fever, cough and loss of sensations of
smell or taste.
The bottom line is that if we define major symptoms
by the need for hospitalization, 95% did not have major symptoms.
Antibodies and Air Pollution
An antibody survey of New York provided data for various regions of
the state.
There is an obviously higher rate of antibody positive
people in the New York City area, and a dramatically lower rate in
rural areas. This could be explained (and will be) by greater
transmission in the city, but also could be due to greater air
pollution in the city.
There are already studies that show, for
example, an association between air pollution and the frequency of
RNA positive tests, and between air pollution and deaths blamed on
COVID-19.
One study estimates,
"An increase of 1 microgram per cubic meter of
fine particulates in the air is associated with an 8% increase in
the COVID-19 death rate in the United States". [16]
Another study
found a similar correlation in China, Italy and the USA using
satellite measures of particulate matter, Carbon Monoxide and
Nitrogen Dioxide. [18]
A study in England correlated COVID-19
lethality with Nitrogen Oxide, Nitrogen Dioxide and Ozone levels.
[19]
An Italian study showed a very high correlation between the number
of times particulate matter limits were exceeded in an area and the
number of infected
(i.e. RNA-positive) people.
Most of the polluted areas, by this
measure, were in northern Italy. [17]
A study in London, England
showed a strong correlation between higher air pollution and higher
numbers of RT-PCR RNA test rates. [20]
Returning to the New York data, the highest fraction of people who
tested antibody positive after volunteering for testing at grocery
stores and community centers was in,
New York City (20%) followed by
Westchester/Rockland (14%) and Long Island (11%)...
The regions with
the lowest fraction testing positive were,
Southern Tier (2.4%),
Capital District (2.2%) and Central NY (1.9%)...
Southern Tier is a
hilly and agricultural area on the southern border of the state.
The
Capital District contains the city of Albany, and is dependent
largely on government, healthcare and education employment.
Central
NY contains the city of Syracuse. While once industrial, most
employment is now in education, research, health care and services.
This evidence is far from proof that false positive antibody tests
can be induced by high levels of air pollution, but given that RNA
positivity and COVID deaths are associated with air pollution, it is
a hypothesis that should be considered.
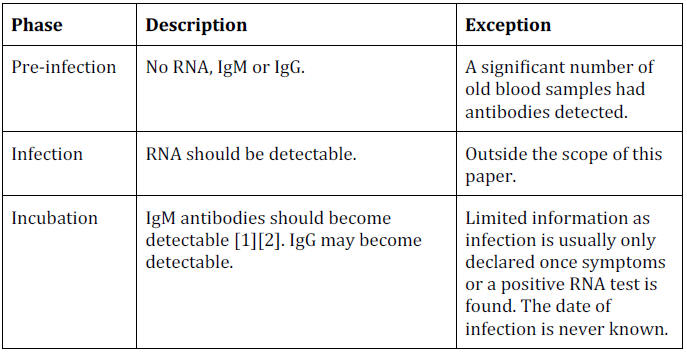
Review of Timeline
Based on the findings in this paper we can review the evidence for
the theoretical timeline.
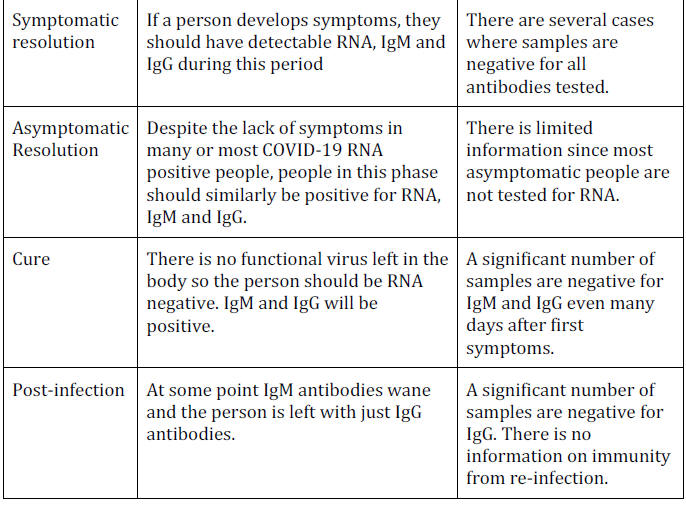
Conclusions
Positive COVID-19 antibody tests have only been found in a minority
of people in the general population even where the virus is believed
to have been circulating for months.
These fractions are generally
taken as truth, but one would expect a highly infectious virus to
have spread much more widely.
There is a lot riding on this data, if
only a small minority of people have COVID-19 IgG antibodies, then
it may be declared by vaccine proponents that natural immunity is
not possible, and that a vaccine may still be necessary, even
mandatory.
The faith in this data is hard to understand since there is no
evidence that the vast majority of people in surveys were ever
'infected' (i.e. were ever RNA positive) and no evidence that the
antibodies seen during the survey were not present in the past.
On
the other hand, there is also no evidence that the majority who test
negative were truly never 'infected' (i.e. never were RNA positive).
Determining immunity is also virtually impossible. Obviously there
would be ethical problems re-challenging people with a virus that is
believed to be fatal in some people.
There are, however, a
significant number of people who test RNA positive after symptoms
have resolved, and after testing RNA-negative.
This could be used as
evidence that re-infection is possible (strengthening the case for a
vaccine) but given that these people are asymptomatic, may just
indicate false positives. [30]
There is no evidence currently that the presence of IgG antibodies
prevents people from becoming RNA positive again or, conversely,
that the absence of IgG antibodies makes people vulnerable to
becoming RNA positive.
Proof that a group without COVID-19 IgG antibodies are more
vulnerable could not just look at the re-occurrence of RNA, because
that usually occurs without symptoms.
Even if occurrence of RNA with
symptoms is more common, one would have to show that the overall
risk of serious illness and death was higher, after adjusting for
baseline differences between the groups with and without IgG
antibodies.
The one experiment that could show whether antibody tests are
actually meaningful would be a time series of a large number of
people who are currently negative on all tests.
This experiment
would be time consuming, inefficient (as many people would never
become positive on any tests), intrusive (frequent nasal swabs and
blood tests) and obviously very expensive.
Those are practical
considerations, but in the absence of such an experiment we are
almost totally in the dark about COVID-19 antibody testing.
Given
the billions being spent on COVID and the trillions being lost by
the economy, it surely is not impossible to do some worthwhile
science.
Additionally, if virus was ever purified from people who were RNA
positive and symptomatic, this could be used to expose animals, and
could be used to detect antibodies that are definitely from
COVID-19, and not just to proteins derived from the putative 30,000
base COVID-19 genome.
References
[1] Chan J F-W et al. A familial cluster of pneumonia associated
with the 2019 novel coronavirus indicating person-to-person
transmission: a study of a family cluster. Lancet. 2020 Jan 24.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30154-9/fulltext
[2] Zhou F et al. Clinical course and risk factors for mortality of
adult inpatients with COVID-19 in Wuhan, China: a retrospective
cohort study. Lancet. 2020 Mar 11.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30566-3/fulltext
[3] Weixel N. FDA changes policy, requires manufacturers to submit
antibody
test data. The Hill.
https://thehill.com/policy/healthcare/495967-fda-changes-policy-to-require-manufacturers-submit-antibody-test-data
[4] qSARS-CoV-2 IgG/IgM Rapid Test. Cellex. 2020 Apr 7.
https://www.fda.gov/media/136625/download
[5] DPP COVID-19 IgM/IgG System. Chembio. 2020 Apr 14.
https://www.fda.gov/media/136963/download
[6] SARS-CoV-2 IgG. Abbott. 2020 Apr 26.
https://www.fda.gov/media/137383/download
[7] Anti-SARS-CoV-2 Rapid Test. Autobio. 2020 Apr 24.
https://www.fda.gov/media/137367/download
[8] SARS-CoV-2 Total Ab. BioRad. 2020 May 1.
https://www.fda.gov/media/137579/download
[9] LIAISON® SARS-CoV-2 S1/S2 IgG. DiaSorin. 2020 Apr 29.
https://www.fda.gov/media/137359/download
[10] Anti-SARS-CoV-2 ELISA (IgG). EuroImmun. 2020 May 4.
https://www.fda.gov/media/137609/download
[11] SARS-CoV Microsphere Immunoassay. Wadsworth Center. 2020 Apr
30. https://www.fda.gov/media/137541/download
[12] VITROS Anti-SARS-CoV-2 IgG. Ortho Clinical Diagnostics. 2020
Apr 24. https://www.fda.gov/media/137363/download
[13] Elecsys Anti-SARS-CoV-2. Roche. 2020 May 2.
https://www.fda.gov/media/137605/download
[14] COVID-19 ELISA IgG Antibody Test. Mount Sinai Laboratory. 2020
Apr 15. https://www.fda.gov/media/137029/download
[15] Slot E et al. Herd immunity is not a realistic exit strategy
during a COVID-19 outbreak. ResearchSquare. 2020 Apr.
https://www.researchsquare.com/article/rs-25862/v1
[16] Wu X et al. Exposure to air pollution and COVID-19 mortality in
the United
States. medRxiv. 2020 Apr 5.
https://www.medrxiv.org/content/10.1101/2020.04.05.20054502v1
[17] Setti L et al. Evaluation of the potential relationship between
Particulate
Matter (PM) pollution and COVID-19 infection spread in Italy. SIMA,
Università di Bologna, Università degli studi di Bari Aldo Moro.
2020 Apr.
https://www.guapo-air.org/sites/default/files/2020-03/Evaluation%20of%20the%20potential%20relationship%20between%20Particulate%20Matter%20%28PM%29%20pollution%20and%20COVID-19%20infection%20spread%20in%20Italy.pdf
[18] Pansini R et al. COVID-19 higher induced mortality in Chinese
regions with
lower air quality. medrxiv. 2020 Apr 7.
https://www.medrxiv.org/content/10.1101/2020.04.04.20053595v2.full.pdf
[19] Travaglio M et al. Links between air pollution and COVID-19 in
England.
medRxiv. 2020 Apr 16.
https://www.medrxiv.org/content/10.1101/2020.04.16.20067405v2
[20] Sasidharan M et al. A vulnerability-based approach to
human-mobility reduction for countering COVID-19 transmission in
London while considering local air quality. medRxiv. 2020 Apr 17.
https://www.medrxiv.org/content/10.1101/2020.04.13.20060798v1
[21] Bryan A et al. Performance Characteristics of the Abbott
Architect SARS-CoV-2 IgG Assay and Seroprevalence Testing in Idaho. medRxiv. 2020 Apr
27. https://www.medrxiv.org/content/10.1101/2020.04.27.20082362v1
[22] Wu X et al. Serological tests facilitate identification of
asymptomatic SARS- CoV-2 infection in Wuhan, China. J Med Virol.
2020 Apr 20.
https://onlinelibrary.wiley.com/doi/10.1002/jmv.25904
[23] Beeler D. PCR and Serological Studies. Google. 2020 May 10
[downloaded].
https://docs.google.com/spreadsheets/d/1zC3kW1sMu0sjnT_vP1sh4zL0tF6fIHbA6fcG5RQdqSc/edit#gid=0
[24] Slot E et al. Herd immunity is not a realistic exit strategy
during a COVID-19
outbreak. ResearchSquare. 2020 Apr.
https://www.researchsquare.com/article/rs-25862/v1
[25] Shi Y et al. Antibody responses against SARS-coronavirus and
its nucleocaspid in SARS patients. J Clin Virol. 2004 Sep; 31(1):
66-8.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7129167/
[26] Why Do We Need Antibody Tests for COVID-19 and How to Interpret
Test Results. Diazyme Laboratories. 2020.
http://www.diazyme.com/covid-19-antibody-tests
[27] Sethuraman N. Interpreting Diagnostic Tests for SARS-CoV-2.
JAMA.
2020 May 6.
https://jamanetwork.com/journals/jama/fullarticle/2765837
[28] To KK et al. Temporal profiles of viral load in posterior
oropharyngeal saliva
samples and serum antibody responses during infection by SARS-CoV-2:
an observational cohort study. Lancet Infect Dis. 2020 May; 20(5):
565-574.
https://www.sciencedirect.com/science/article/pii/S1473309920301961
[29] Xiang F et al. Antibody Detection and Dynamic Characteristics
in Patients with COVID-19. Clin Infect Dis. 2020 Apr 19.
https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa461/5822173
[30] Crowe D. Flaws in Coronavirus Pandemic Theory. The Infectious
Myth.
2020 Mar.
http://theinfectiousmyth.com/book/CoronavirusPanic.pdf
[31] Wu F et al. Neutralizing antibody responses to SARS-CoV-2 in a
COVID-19 recovered 2 patient cohort and their implications. medRxiv.
2020 Mar 30.
https://www.medrxiv.org/content/medrxiv/early/2020/04/06/2020.03.30.20047365.full.pdf
[32] Stickler A. Life sentence. BBC News. 2008 Jul 28.
http://news.bbc.co.uk/today/hi/today/newsid_7523000/7523680.stm
[33] EUA Authorized Serology Test Performance. FDA. 2020 May 6
[accessed].
https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance
|