|
the presence of a gut microbiome very early in life affects the brain development
and
adult social behavior of zebra fish. suggests that gut microbes can have a crucial early influence on the brain's social development...
Scientists found this influence in fish, but molecular and neurological evidence plausibly suggests that some form of it could also occur in mammals, including humans.
In a paper published in early November in PLOS Biology, researchers found that zebra fish who grew up lacking a gut microbiome were far less social than their peers with colonized colons, and the structure of their brains reflected the difference.
In a related article in BMC Genomics in late September, they described molecular characteristics of the neurons affected by the gut bacteria.
Equivalents of those neurons appear in rodents, and scientists can now look for them in other species, including humans.
In recent decades, scientists have come to understand that the gut and the brain have powerful mutual influences. Certain types of intestinal ulcers, for example, have been linked to worsening symptoms in people with Parkinson's disease.
And clinicians have long known that gastrointestinal disorders are more common in people who also have neuro-developmental disorders, such as ADHD and autism spectrum disorder.
How these anatomically separate organs exert their effects, however, is far less clear.
Philip Washbourne, a molecular biologist at the University of Oregon and one of the principal co-authors of the new studies, has been studying genes implicated in autism and the development of social behaviors for over two decades.
But he and his lab were looking for a new model organism, one that displayed social behavior but was quicker and easier to breed than their go-to, mice.
Germ-Free Fish
Zebra fish, which are also widely used in genetics research, reproduce quickly and are naturally social.
They are also transparent until adulthood, which allows researchers to observe their internal development without having to dissect them - a feat that is all but impossible in mammalian models, such as mice.
The team began experimenting with embryos from a line of "germ-free" zebra fish bred to lack a gut microbiome.
After the tiny fish hatched, the researchers immediately inoculated some of them with a healthy mix of gut bacteria. But they waited a full week before inoculating the remaining fish, forcing them to begin their development with a blank slate.
The fish that had been inoculated at birth began shoaling right on schedule, at roughly 15 days old.
But when it came time for the germ-free fish to start,
Even though the fish had been dosed retroactively with gut microbes, they weren't hitting the same social development milestones as their peers.
When Eisen, Washbourne and their team examined the fishes' brains, they discovered obvious structural differences.
In the fish that spent their first week of life without a microbiome, a specific cluster of forebrain neurons that affect social behavior showed more interconnections.
The cluster also had significantly fewer microglia, the neural immune cells responsible for cleaning up detritus in the brain.
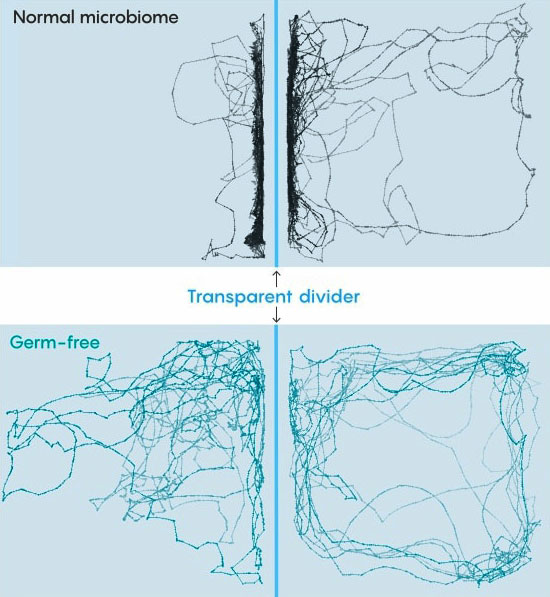
Lines trace the paths of zebra fish swimming in a special experimental tank. Fish that grew up with a normal microbiome spent most of their time near a transparent divider to stay near fish on the other side. Fish that were "germ free" for their first week
were less sociable and swam more randomly. Source: doi.org/10.1371/journal.pbio.3001838
The team hypothesized that a healthy gut microbiome somehow enables microglia to flourish in zebra fish brains.
Then, during certain critical developmental periods, the microglia act like maintenance workers, pruning the wildly branching "arms" on neurons.
Without microglia to trim them back, the germ-free fishes' social neurons became tangled and overgrown like an untended bramble.
How the gut microbes send signals to the fishes' developing brains to produce these effects isn't clear. Bacteria release a staggering array of chemicals, and any sufficiently small compound could theoretically cross the blood-brain barrier.
But it's also possible that immune cells moving between the gut and the brain carry signal molecules with them, or that certain signals travel up from the gut along the vagus nerve.
Many Sociable Species
Similar mechanisms may be at play in the social development of other vertebrates, including humans.
Social grouping is a common survival strategy across the animal kingdom.
In fact, Washbourne and Eisen had previously identified nearly identical social neurons in mice.
When zebra fish are about two weeks old,
they typically begin to socialize in groups called shoals.
Morais cautioned, however, that neither zebra fish nor mice are perfect analogues for human beings - or for each other.
Nevertheless, the principle could be broadly true for diverse groups of organisms.
It's possible that different microbial chemicals could still influence microglial abundance in the brains of zebra fish, mice, humans and other animals, said Eisen.
But she agrees that it's dangerous to unequivocally conflate different species.
Model organisms,
A Multiplicity of Microbiomes
In the future, Eisen, Washbourne and their teams want to pinpoint exactly how the zebra fish's gut microbes send signals to its brain.
They also want to establish how long the sensitive period for neurodevelopment is, to see whether early intervention in the gut can put brain development back on track.
Eventually, they hope that this research will provide a deeper understanding of how neuro-developmental disorders arise in people - though this may prove difficult.
The logistics of designing a clinical trial to test gut interventions in human infants would be tough because conditions like autism spectrum disorder aren't usually diagnosed until age 7 or later, likely long after the critical window has closed.
Microbiomes also vary significantly even between individuals of the same species.
Simply looking at a person's microbiome isn't a useful diagnostic tool for neuro-developmental disorders.
For Washbourne, if this sensitive developmental period exists in humans, it could make intervention nearly impossible.
But even being able to characterize the gut's effect on the brain in some small way helps unravel a deeply complex human mystery.
For now, he said, that's enough...
|