|
ABSTRACT
And I present evidence for the controlled-demolition hypothesis, which is suggested by the available data, and can be tested scientifically, and yet has not been analyzed in any of the reports funded by the US government.
A video clip provides eye-witness evidence regarding this metal at ground zero:
The photographs below by Frank Silecchia show chunks of the hot metal being removed from the North Tower rubble on September 27, 2001 (according to photographer’s aid). Notice the color of the lower portion of the extracted metal—this tells us much about the temperature of the metal and provides important clues regarding its composition, as we shall see.
Next, as a basis for discussion, I invite you to consider the collapse of the 47-story WTC 7, which was never hit by a jet. Here is the building prior to and on September 11, 2001:
WTC 7: 47 - Story, steel-frame building..
WTC 7 on
9-11-01. WTC 7 is the tall sky-scraper in the background, right.
WTC 7 collapsed completely, onto its own footprint
Now that you have seen the still photographs, it is important to the discussion which follows for you to observe video clips of the collapse of this building, so go to:
Then consider a video close-up of the same building, southwest corner, as this corner begins its steady drop to the ground:
New, side-by-side comparison of WTC7 collapse and a controlled demolition using explosives:
What did you observe?
Note that references to web pages are used in this paper due largely to the importance of viewing motion picture clips, thus enhancing consideration of the laws of motion and physics generally. High-quality photographs showing details of the collapses of WTC 7 and the WTC Towers can be found in books (Hufschmid, 2002; Paul and Hoffman, 2004), magazines (Hoffman, 2005; Baker, 2005) and at http://911research.wtc7.net/wtc/evidence/photos/collapses.html .
The goal is to promote further
scrutiny of the official government-sponsored reports as well as
serious investigation of the controlled-demolition hypothesis. (No
rebuttal of my argument can be complete, of course, unless it
addresses all of these points.)
Thirteen Reasons to Challenge Government-sponsored 1. Molten Metal: Flowing and in Pools
The existence of molten metal at Ground Zero was reported by several observers (see first photograph above), including Greg Fuchek:
Sarah Atlas was part of New Jersey’s Task Force One Urban Search and Rescue and was one of the first on the scene at Ground Zero with her canine partner Anna. She reported in Penn Arts and Sciences, summer 2002, ‘Nobody’s going to be alive.’ Fires burned and molten steel flowed in the pile of ruins still settling beneath her feet. (Penn, 2002; emphasis added.)
Notice that the molten metal (probably not steel alone; see discussion below) was flowing down in the rubble pile early on; so it is not the case that the molten metal pools formed due to subterranean fires after the collapses.
The observer notes that the observed surface of this metal is still reddish-orange some six weeks after 9-11. This implies a large quantity of a metal with fairly low heat conductivity and a relatively large heat capacity (e.g., iron is more likely than aluminum) even in an underground location. Like magma in a volcanic cone, such metal might remain hot and molten for a long time—once the metal is sufficiently hot to melt in large quantities and then kept in a fairly-well insulated underground location.
Moreover, as
hypothesized below, thermite reactions may well have resulted in
substantial quantities (observed in pools) of molten iron at very
high temperatures – initially above 2,000 °C (3,632 °F). At these
temperatures, various materials entrained in the molten metal pools
will continue to undergo exothermic reactions which would tend to
keep the pools hot for weeks despite radiative and conductive
losses. Any thermite cutter charges which did not ignite during the
collapse would also contribute to the prolonged heating.
Thermite contains its own supply of oxygen and so the reaction cannot be smothered, even with water. Use of sulfur in conjunction with the thermite, for example in thermate, will accelerate the destructive effect on steel, and sulfidation of structural steel was indeed observed in some of the few recovered members from the WTC rubble, as reported in Appendix C of the FEMA report. (FEMA, 2002; see also here)
On the other hand, falling buildings (absent incendiaries such as thermite) have insufficient directed energy to result in melting of large quantities of metal; any particles of molten metal somehow formed during collapse will not coalesce into molten pools of metal!
Metals expert Dr. Frank Gayle (working with NIST) stated:
And in an a fact sheet released in August, 2006, NIST states:
None of the official reports tackles the mystery of the molten metal pools. Yet this is clearly a significant clue to what caused the Towers and WTC 7 to collapse. So an analysis of the composition of the previously-molten metal is required by a qualified scientific panel. This could well become an experiment crucis.
We will return to the question of fire-induced stresses and WTC collapses later.
Note that the approximate temperature of a hot metal is given by its color, quite independent of the composition of the metal.
(A notable exception is falling liquid aluminum, which due to low emissivity and high reflectivity appears silvery-gray in daylight conditions, after falling through air 1-2 meters, regardless of the temperature at which the poured-out aluminum left the vessel. Aluminum does incandesce (glow) like other metals, but faintly, so that with the conditions described in the previous sentence (which prevailed at the WTC on 9/11), falling liquid aluminum will appear silvery-gray. Rapid oxidation of the hot flowing aluminum will contribute to the observed appearance. [Experiments: Jones, 2006])
We see from the photograph above that solid metal from the WTC rubble existed at salmon-to-yellow-hot temperature (approx. 1550 - 1900 oF, 845 - 1040 oC.) The temperature is well above the melting temperatures of lead, zinc and aluminum, and these metals can evidently be ruled out since they would be runny liquids at much lower (cherry-red or below) temperatures.
However, the observed hot
specimen could be structural steel (from the building) or iron (from
a thermite reaction) or a combination of the two. Additional
photographs of the hot metal could provide further information and
advance the research.
The abundance of iron (as opposed to aluminum) in this material is indicated by the reddish rust observed. When a sample is obtained, a range of characterization techniques will quickly give us information we seek. X-ray energy dispersive spectrometry (XEDS) will yield the elemental composition, and electron energy-loss spectroscopy will tell us the elements found in very small amounts that were undetectable with XEDS.
Electron-backscattered diffraction in the scanning electron microscope will give us phase information; the formation of certain precipitates can tell us a minimum temperature the melt must have reached. We will endeavor to obtain and publish these data, whatever they reveal.
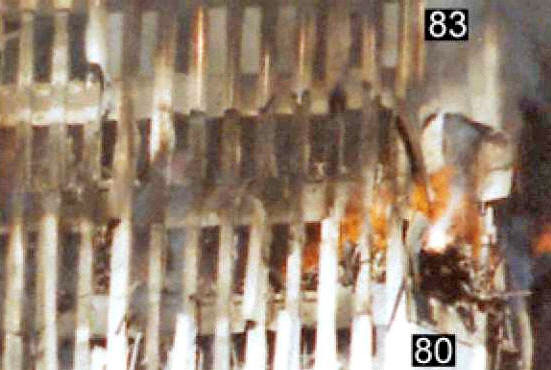
Click below image to see video. Regarding this photo, NIST states:
Thus it is established that the “glowing liquid” flow is associated spatially and temporally with the “bright spot” observed on the corner of the 80th floor of WTC 2. The photograph below shows, for comparison, a thermite reaction with a white aluminum-oxide dust plume extending from very bright reaction region.
(Experiment by the author and colleagues in which thermite-plus-sulfur cut through a steel cup in a fraction of a second. Any thermite reaction is a dangerous reaction and should only be performed by a trained professional capable of assessing the hazards and risks.)
The similarities between the known thermite reaction and the hitherto unknown reaction at the WTC Tower are plain to see. These discoveries strongly motivate an immediate in-depth investigation of the use of thermite-type reactions in the destruction of the World Trade Center on 9/11/2001.
Dramatic footage reveals yellow-to-white hot molten metal dripping from the South WTC Tower at this SAME CORNER just minutes before its collapse. (Watch above related video):
Is the falling molten metal from WTC Tower 2 (Top photos) more likely molten iron from a thermite reaction (lower left) OR pouring molten aluminum (lower right)?
Thus, molten aluminum is already ruled out with high probability. But molten iron with the characteristics seen in this video is in fact consistent with a thermite-reaction attacking the steel columns in the Tower, thus weakening the building just prior to its collapse, since thermite produces molten iron at yellow-to-white hot temperatures.
(As some of the molten metal hits the side of the building in the video clip above, the white-hot interior is evidently exposed as the metal “splashes”.)
Also, the fact that the liquid metal retains an orange
hue as it nears the ground (right photograph) further rules out
aluminum, and suggests a mid-flight thermite reaction (typical of thermite).
The absence of dark smoke trailing behind the falling liquid material indicated it was not fuel-soaked debris. Indeed, white ash is seen in these videos trailing away from the falling liquid material. Falling molten steel would not produce such a white ash, whereas thermites produce a white aluminum-oxide ash which indeed trails away from the falling molten metal generated in the reaction, corresponding to the observations.
A memorial constructed from structural steel from the WTC Towers located at Clarkson University in Potsdam, New York, is the source of previously-molten metal samples. Porous, solidified splatter found with the compacted dirt from this memorial is being analyzed. Results from these studies were presented at the 2006 meeting of the Utah Academy of Science followed by the American Scholars Symposium (Los Angeles), and are made available here.
Further strong evidences for the use of aluminothermics continue to be discovered in our analyses and will be reported in a separate paper.
There were in fact no “violent thermite” reactions seen. We observed that the temperature of the molten aluminum in contact with the rusty iron simply cooled at about 25 oC per minute (measured with an infrared probe) until the aluminum solidified, so that any thermite reactions between the aluminum and iron oxide must have been minimal and did not compete with radiative and conductive cooling, thus NOT supporting predictions made by Greening. There was no observable damage or even warping of the steel. (See photograph below.)
Nor were violent reactions observed when we dropped molten aluminum onto crushed gypsum and concrete (wet or dry) and rusty steel. [Jones, 2006; available here]. These experiments lend no support whatever to the notion [see Greening, 2006] that molten aluminum in the WTC Towers could have destroyed the enormous steel columns in the cores of the buildings, even if those columns were rusty and somehow subjected to direct contact with liquid aluminum.
Note that the latest proposed explanation provides no mechanism for feeding fuel (office materials) into the oxygen stream, i.e., this is not like an oxy-acetylene torch. Moreover, even if the tanks survived the plane crashes, to melt steel would require steel (not air) temperatures of over 2,700 degrees F – while the steel structure is wicking the heat away from the heat source. Greening needs to consider heat transport in the steel as well as the probability that oxygen tanks in the planes could survive the destructive crashes of the planes.
Finally, no plane hit WTC 7, so this latest hypothesis fails from the outset in this case. But we do consider alternative hypotheses such as these. Finally, the data from the solidified slag are not consistent with molten structural steel since it contains almost no chromium, yet shows significant fluorine and elemental sulfur, and high concentrations of nickel and zinc. These results will be the subject of a separate paper.
A brief discussion of recent results, presented at the Utah Academy of Sciences and subsequent colloquia is available here.
(Recall also that the yellow color of the molten metal (video clip above) implies a temperature of approximately 1100oC—too high for the dark-smoke hydrocarbon fires burning in the building.)
This is a point worth emphasizing: aluminum has low emissivity and high reflectivity, so that in daylight conditions after falling through air 1-2 meters, molten aluminum will appear silvery-gray, while molten iron (with its characteristic high emissivity) will appear yellow-white (at ~1100oC) as observed in the molten metal dripping from the South Tower just before its collapse (see: http://www.supportthetruth.com/jones.php). We also recall that this molten metal, after falling approximately 150 meters (or yards) still retained a reddish orange color (photograph above). This is not the behavior of falling, molten aluminum.
Molten aluminum poured onto rusted steel: silvery flow, and no violent reactions observed at all.
NIST states the hypothesis that flowing aluminum with partially burned organic materials mixed in, “can display an orange glow.” But will it really do this? I decided to do an experiment to find out. Our group melted aluminum in a steel pan using an oxy-acetylene torch. Then we added plastic shavings—which immediately burned with a dark smoke, as the plastic floated on top of the hot molten aluminum. Next, we added wood chips (pine, oak and compressed fiber board chips) to the liquid aluminum.
Again, we had fire and smoke, and again, the hydrocarbons floated on top as they burned. We poured out the aluminum and all three of us observed that it appeared silvery, not orange! We took photos and videos, so we will have the recorded evidence as these are processed. Of course, we saw a few burning embers, but this did not alter the silvery appearance of the flowing, falling aluminum.
Videos of our experiments involving organics added to liquid aluminum are available here.
We demonstrated that the thermite reaction would not ignite at this high temperature. Later, the thermite reaction was triggered by burning a magnesium strip in contact with the thermite. An electrical superthermite “match” could have been used and remotely triggered via radio signal.
Thermite did not ignite when
heated with a propane torch. “Superthermites” use tiny particles of aluminum known as “nanoaluminum” (<120 nanometers) in order to increase their reactivity. Explosive superthermites are formed by mixing nanoaluminum powder with fine metal oxide particles such as micron-scale iron oxide dust.
Based on these and other discoveries, the possible use of incendiary thermites and explosive superthermites on 9/11 should be investigated immediately and vigorously.
Workers evidently peering into the hot “core” under the WTC rubble. (See related BBC News). For recorded eyewitness testimony of the molten metal pools under both Towers and WTC 7, see here.
It would be interesting if underground fires could somehow produce large pools of molten steel, for example, but then there should be historical examples of this effect since there have been many large fires in numerous buildings. It is not enough to argue hypothetically that fires could possibly cause all three pools of orange-hot molten metal.
In addition, the use of explosives such as HMX or RDX should also be considered. “Superthermites” are also explosive as must be remembered in any in-depth investigation which considers hypotheses suggested by the available data. The official reports by NIST, FEMA and the 9-11 Commission strikingly omit mention of large quantities of molten metal observed in the basement areas of WTC 7 and the Towers. The fact that the official reports do not adequately address the issue of molten metal found at the sites provides compelling motivation for continued research on the WTC collapses.
|