|
holds a bottle of Ivermectin in Colombia on July 21, 2020. (Luis Robayo/AFP via Getty Images)
However, since the onset of the COVID-19
'pandemic', the National
Institutes of Health (NIH) and affiliated health authorities have
vociferously recommended against Ivermectin as a potential treatment
for the virus. Though the Food and Drug Administration (FDA) has approved Ivermectin for human use in treating conditions caused by parasites, it has also insisted that Ivermectin,
In a social media message that has gone viral, the FDA labeled it as a drug for horses and not fit for human consumption:
The post made headlines and was one of the FDA's most successful social media campaigns.
A growing body of research shows that Ivermectin,
Many doctors have praised the drug for its broad yet effective,
...properties.
Ivermectin - Antiparasitic Beginnings
Ivermectin made its name through its significant benefits in treating parasitic infections.
In 1973, Satoshi Omura and William C. Campbell, working with the Kitasato Institute in Tokyo, found an unusual type of Streptomyces bacteria in Japanese soil near a golf course.
In laboratory studies, Omura and Campbell discovered that this Streptomyces bacteria could cure mice infected with the roundworm Heligmosomoides polygyrus. Campbell isolated the bacteria's active compounds, naming them avermectins, and the bacteria was thus called S. avermitilis.
Despite decades of searching worldwide, researchers have yet to find another microorganism that can produce avermectin.
It was changing one of the bonds of avermectin through a chemical process that produced Ivermectin, which was proven successful in treating onchocerciasis and lymphatic filariasis, both of which are debilitating diseases common in the developing world.
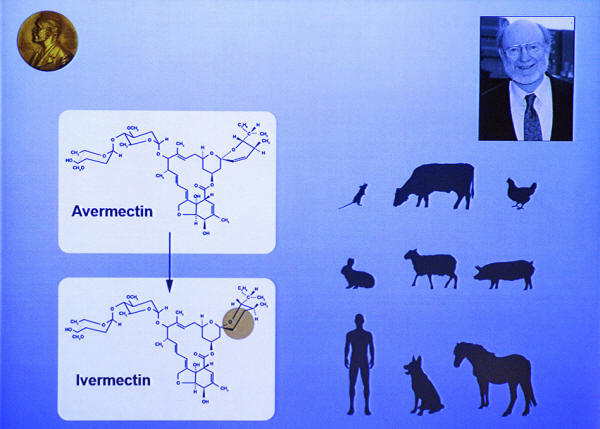
A portrait of William Campbell and an illustration describing his work displayed on a screen during a press conference of the 2015 Nobel Medicine Prize. William Campbell and Satoshi Omura won the Nobel Medicine Prize for their discoveries of treatments against parasites - Avermectin, which was modified to Ivermectin.
(JONATHAN NACKSTRAND/AFP via Getty Images)
Though its broad antiparasitic functions are not well understood, it is known that Ivermectin penetrates parasites' nervous systems, turning off their neurons' actions, possibly deactivating and killing them.
As part of a donation campaign launched in 1988 by Merck & Co., Inc., the manufacturer of Ivermectin, the drug was used in Africa to treat river blindness.
Also called onchocerciasis, river blindness is a tropical disease caused by Onchocerca volvulus worms.
It is the second-most common cause worldwide of infectious blindness.
The Onchocerca worms mature in the skin of an infected individual ("the host").
After mating, female worms can release into the host's skin up to 1,000 microfilariae a day; the female worms live for 10 to 14 years. The presence of these worms can lead to scarring in the tissues and, when microfilariae invade the eye, can cause visual impairment or complete loss of vision.
The World Health Organization estimates that 18 million people are infected globally, and 270,000 have been blinded by onchocerciasis.
When Merck distributed Ivermectin in areas hardest hit by the disease, treatment benefited the residents' overall health and led to economic recovery.
Ivermectin replaced previous drugs that had devastating side effects.
Ivermectin has also proven effective against lymphatic filariasis, known as elephantiasis.
Parasitic worms transmitted through the bite of an infected mosquito can grow and develop in lymphatic vessels, which regulate the body's water balance. When certain vessels are blocked, the areas - typically the legs and genitals - can swell, with the legs enlarging to elephant-like stumps.
Worldwide, more than 120 million people are infected, 40 million of whom are seriously incapacitated and disfigured.
The World Health Organization listed Ivermectin as an essential drug and has advised many countries to run annual campaigns to rid people of these parasites. Such recommendations are a solid testament to Ivermectin's safety.
For their work, including the discovery of avermectin, in 2015, Omura and Campbell were among three recipients of the Nobel Prize in Physiology or Medicine.
It is an indispensable drug for the underdeveloped world, with about 3.7 billion doses administered as part of global campaigns during the past 30 years.
To this day, Ivermectin remains a staple drug of tropical areas and an essential drug in treating onchocerciasis, lymphatic filariasis, strongyloidiasis, and scabies.
Ivermectin and COVID-19
Analyses of studies on Ivermectin have found it effective as a prevention, a treatment for acute COVID-19, and in advanced stages of infection by the virus.
The Changing Public Health Messaging on Ivermectin
The NIH's stance on Ivermectin has changed several times.
Early in the 'pandemic', there was little information on Ivermectin as a potential treatment for the virus.
Researchers administered Ivermectin to SARS-CoV-2-infected monkey kidney cells in the laboratory and found the drug beneficial in very high doses.
However, the researchers concluded that further study was needed.
Many health agencies, including the NIH, the CDC, and other global health regulators concluded that Ivermectin could kill the virus only at toxic levels.
Even now, NIH's statement on Ivermectin for COVID-19 reads:
In October 2020, the first clinical study showing the benefits of Ivermectin was published by the journal CHEST.
The study's lead author, Dr. Jean-Jacques Rajter, is a critical care doctor specializing in pulmonary medicine.
Rajter gave a testimony (pdf) of his findings to the Senate Committee on Homeland Security & Governmental Affairs in December 2020.
The day after he saw the Australian study, one of his COVID patients dramatically deteriorated from breathing normally at room oxygen levels to requiring intubation. The patient's son pleaded with Rajter to save his mother using whatever options available.
Rajter recognized that Hydroxychloroquine would be ineffective in the advanced stages of COVID.
After much deliberation, he tried Ivermectin.
More clinical studies were published, showing the benefits of Ivermectin as a prophylactic treatment. (pdf 1, pdf 2).
The findings encouraged the use of Ivermectin among doctors desperate to find a cure.
Meanwhile, by October 2020, research into COVID-19 vaccines and the use of remdesivir to treat the virus was already in full swing.
According to the FDA, specific criteria should be met for the EUA (Emergency Use Authorization) to be granted for vaccines and medications, including that there are,
Some doctors say that if Ivermectin's use for COVID had been approved, it would have made the EUAs for vaccines and remdesivir null and void.
Following the Australian study, the FDA published a statement, "FAQ: COVID-19 and Ivermectin Intended for Animals," highlighting the use of Ivermectin in animals and advising against the use of Ivermectin for COVID-19.
The NIH also discouraged the use of Ivermectin, albeit briefly. On Jan. 14, 2021, the NIH changed its statement, writing that there was no evidence to recommend or disapprove the use of Ivermectin.
However, in April 2022, the statement changed to strongly disapproving of using Ivermectin.
Health Authority Overreach
Despite the NIH's neutral statement on Ivermectin for most of 2021, the FDA actively campaigned against using Ivermectin in COVID-19 patients.
On Aug. 26, 2021, the CDC sent an emergency warning against using Ivermectin; a few weeks later, the American Medical Association and affiliated associations called for an end to Ivermectin use.
Many doctors were thus discouraged from using Ivermectin, and pharmacies refused to prescribe it.
State health agencies warned against (below Twitter) using Ivermectin, and medical boards removed the medical licenses of doctors who prescribed Ivermectin, alleging misinformation.
Yet using the FDA's statement against Ivermectin to ban its use in COVID-19 cases would be considered an overreach.
Since the FDA approved Ivermectin in 1996, this made the drug acceptable for off-label use.
As an ironic side effect of the messaging on Ivermectin, people suddenly found themselves unable to access Ivermectin, and some turned to veterinary-grade Ivermectin.
Though veterinary Ivermectin is the same product as medicinal Ivermectin, the manufacturing standard is not the same as it is for human-grade pharmaceuticals.
Contradictory Research and Campaigns
Though the initial research in 2020 showed promising results for Ivermectin, published studies reported conflicting findings by the following year.
The NIH has funded many studies on the effectiveness of Ivermectin, the most recent being ACTIV-6.
Individuals can participate in the study once they develop COVID by selecting Ivermectin from four other drugs. The drug was sent to them via mail. This method means that some people in the study could have recovered by the time they received the Ivermectin.
There are some controversies regarding this study.
The first is that the authors changed the primary endpoints during the study, which is heavily frowned upon as it can affect the validity and reliability of the outcome.
Initially, the primary endpoint was the number of deaths, hospitalizations, and symptoms reported at day 14.
This was changed to the number of deaths, hospitalizations, and symptoms by day 28. In the actual published study, there was another change, with the endpoint being duration of COVID-19 symptoms.
A rapid review published by the Massachusetts Institute of Technology (MIT) implied that the endpoints were changed because, by the time the study commenced, there were far fewer events of death and hospitalizations; as a result, there would not be enough data for a reliable comparison.
Indeed, the data at the ACTIV-6 livestream showed that the Ivermectin group reported only one death:
There were also further implementations in the study that could impact the observed effectiveness of the drug.
On average, this study's participants received treatment six days after first reporting symptoms. Patients needed to report eligible symptoms and test positive for COVID-19 before receiving drugs.
Due to this added time, about seven percent of the participants had no symptoms by the time Ivermectin arrived.
Despite these negative findings for Ivermectin, there is still some evidence that may demonstrate that Ivermectin can be useful in treating COVID-19.
In the abstract, the authors concluded that taking Ivermectin had "a posterior probability of benefit of .91," this is another way of writing that,
The percent of probability is below 95 percent, making the benefit of Ivermectin insignificant.
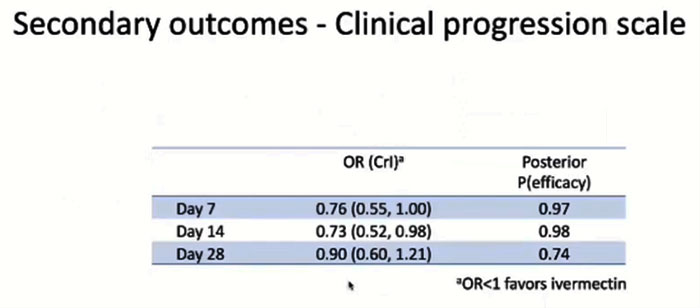
Slides from the ACTIV-6 presentation on a statistically significant secondary endpoint findings.
Another secondary endpoint showed that by day 14, Ivermectin already had a statistically significant 27 percent benefit with 98 percent probability of efficacy.
The FDA and NIH did not respond for comments by press time...
|