|

by Carl Zimmer
July 16,
2013
from
QuantaMagazine Website
The human eye evolved gradually,
with natural selection favoring intermediate forms,
but studies indicate that
complexity may also emerge by other means.
Suren Manvelyan
Scientists are
exploring
how organisms
can evolve elaborate structures
without
Darwinian selection...
In Brief
-
Conventional wisdom holds that complex
structures evolve from simpler ones,
step-by-step, through a gradual evolutionary
process, with Darwinian selection favoring
intermediate forms along the way.
-
But recently some scholars have proposed that
complexity can arise by other means - as a side
effect, for instance - even without natural
selection to promote it.
-
Studies suggest that random mutations that
individually have no effect on an organism can
fuel the emergence of complexity in a process
known as constructive neutral evolution.
Charles Darwin was not yet 30 when he got the
basic idea for the theory of evolution.
But it wasn't until he
turned 50 that he presented his argument to the world.
He spent those two
decades methodically compiling evidence for his theory and
coming up with responses to every skeptical counterargument he
could think of.
And the
counterargument he anticipated most of all was that the gradual
evolutionary process he envisioned could not produce certain
complex structures.
Consider the human eye:
It is made up of
many parts - a retina, a lens, muscles, jelly, and so on -
all of which must interact for sight to occur.
Damage one part -
detach the retina, for instance - and blindness can follow.
In fact, the eye
functions only if the parts are of the right size and shape to
work with one another. If Darwin was right, then the complex eye
had evolved from simple precursors.
In
On The Origin of Species,
Darwin wrote that this idea,
"seems, I freely
confess, absurd in the highest possible degree."
But Darwin could
nonetheless see a path to the evolution of complexity. In
each generation, individuals varied in their traits.
Some variations
increased their survival and allowed them to have more
offspring. Over generations those advantageous variations would
become more common - would, in a word, be "selected."
As new variations
emerged and spread, they could gradually tinker with anatomy,
producing complex structures.
The human eye, Darwin
argued, could have evolved from a simple light-catching patch of
tissue of the kind that animals such as flatworms grow today.
Natural selection
could have turned the patch into a cup that could detect the
direction of the light.
Then, some added
feature would work with the cup to further improve vision,
better adapting an organism to its surroundings, and so this
intermediate precursor of an eye would be passed down to future
generations.
And, step-by-step,
natural selection could drive this transformation to increased
complexity because each intermediate form would provide an
ad–vantage over what came before.
Darwin's musings on the origin of complexity have found
support in modern biology.
Today biologists can
probe the eye and other organs in detail at the molecular level,
where they find immensely complex proteins joining together to
make structures that bear a striking resemblance to portals,
conveyor belts and motors.
Such intricate
systems of proteins can evolve from simpler ones, with natural
selection favoring the intermediates along the way.
But recently some
scientists and philosophers have suggested that complexity can
arise through other routes.
-
Some argue that
life has a built-in tendency to become more complex over
time.
-
Others maintain
that as random mutations arise, complexity emerges as a side
effect, even without natural selection to help it along.
Complexity, they say, is
not purely the result of millions of years of fine-tuning through
natural selection - the process that Richard Dawkins
famously dubbed "the
blind watchmaker."
To some extent, it 'just
happens'...
A Sum of
Varied Parts
Biologists and philosophers have pondered the evolution of
complexity for decades, but according to
Daniel W. McShea, a
paleobiologist at Duke University, they have been hobbled by vague
definitions.
"It's not just that
they don't know how to put a number on it. They don't know what
they mean by the word," McShea says.
McShea has been
contemplating this question for years, working closely with
Robert N. Brandon, also at
Duke.
McShea and Brandon
suggest that we look not only at the sheer number of parts making up
living things but at the types of parts.
Our bodies are made
of 10 trillion cells. If they were all of one type, we would be
featureless heaps of protoplasm.
Instead we have muscle
cells, red blood cells, skin cells, and so on.
Even a single organ can
have many different cell types. The retina, for example, has about
60 different kinds of neurons, each with a distinct task. By this
measure, we can say that we humans are, indeed, more complex than an
animal such as a sponge, which has perhaps only six cell types.
One advantage of this definition is that you can measure complexity
in many ways. Our skeletons have different types of bones, for
example, each with a distinctive shape.
Even the spine is made up
of different types of parts, from the vertebrae in the neck that
hold up our head to the ones that support our rib cage.
In their 2010 book
Biology's First Law, McShea and
Brandon outlined a way that complexity defined in this way could
arise. They argued that a bunch of parts that start out more or less
the same should differentiate over time.
Whenever organisms
reproduce, one or more of their genes may mutate. And sometimes
these mutations give rise to more types of parts.
Once an organism has more
parts, those units have an opportunity to become different. After a
gene is accidentally copied, the duplicate may pick up mutations
that the original does not share.
Thus, if you start with a
set of identical parts, according to McShea and Brandon, they will
tend to become increasingly different from one another. In other
words, the organism's complexity will increase.
As complexity arises, it may help an organism survive better or have
more offspring. If so, it will be favored by natural selection and
spread through the population.
Mammals, for example,
smell by binding odor molecules to receptors on nerve endings in
their nose. These receptor genes have repeatedly duplicated over
millions of years. The new copies mutate, allowing mammals to smell
a wider range of aromas.
Animals that rely heavily
on their nose, such as mice and dogs, have more than 1,000 of these
receptor genes. On the other hand, complexity can be a burden.
Mutations can change the
shape of a neck vertebra, for instance, making it hard for the head
to turn. Natural selection will keep these mutations from spreading
through populations. That is, organisms born with those traits will
tend to die before reproducing, thus taking the deleterious traits
out of circulation when they go.
In these cases,
natural selection works against complexity.
Unlike standard evolutionary theory, McShea and Brandon see
complexity increasing even in the absence of natural
selection. This statement is, they maintain, a fundamental law
of biology - perhaps its only one.
They have dubbed it the
zero-force evolutionary law.
The Fruit-Fly
Test
Recently McShea and Leonore Fleming, a graduate student at
Duke, put the zero-force evolutionary law to the test.
The subjects were
Drosophila flies...
For more than a century
scientists have reared stocks of the flies to use in experiments. In
their laboratory homes, the flies have led a pampered life, provided
with a constant supply of food and a steady, warm climate.
Their wild relatives,
meanwhile, have to contend with starvation, predators, cold and
heat. Natural selection is strong among the wild flies, eliminating
mutations that make flies unable to cope with their many challenges.
In the sheltered
environment of the labs, in contrast, natural selection is feeble.
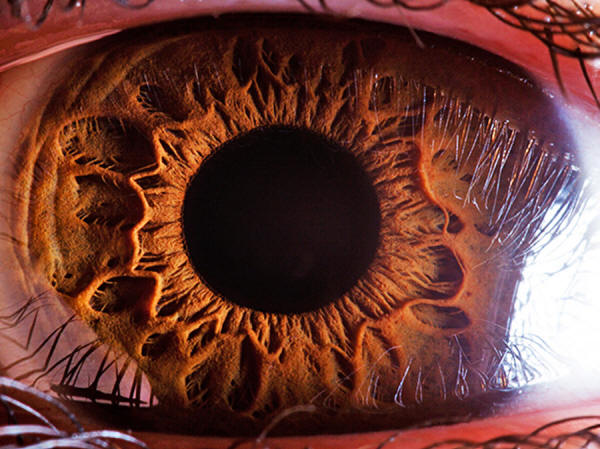
Lab-raised fruit flies are more complex than
wild ones because their sheltered environment
allows even disadvantageous mutations to spread.
This
mutant Drosophila has bar-shaped eyes that are
smaller than normal.
Edward Kinsman
The zero-force evolutionary law makes a clear prediction:
over the past century
the lab flies should have been less subject to the elimination
of disadvantageous mutations and thus should have become more
complex than the wild ones.
Fleming and McShea
examined the scientific literature for 916 laboratory lines of
flies.
They made many different
measures of complexity in each population. In the journal Evolution
& Development, they recently reported (Drosophila
Mutants suggest a strong drive toward Complexity in Evolution)
that the lab flies were indeed more complex than wild ones.
Although some biologists have endorsed the zero-force evolutionary
law,
Douglas Erwin, a leading
paleontologist at the Smithsonian National Museum of Natural
History, thinks it has some serious flaws.
"One of its basic
assumptions fails," he argues.
According to the
law, complexity may increase in the absence of selection.
But that would be true
only if organisms could actually exist beyond the influence of
selection. In the real world, even when they are pampered by the
most doting of scientists, Erwin contends, selection still exerts a
force.
For an animal such as a
fly to develop properly, hundreds of genes have to interact in an
elaborate choreography, turning one cell into many, giving rise to
different organs, and so on.
Mutations may disrupt
that choreography, preventing the flies from becoming viable adults.
An organism can exist without external selection - without the
environment determining who wins and loses in the evolutionary race
- but it will still be subject to internal selection, which takes
place within organisms.
In their new study,
McShea and Fleming do not provide evidence for the zero-force
evolutionary law, according to Erwin,
"because they only
consider adult variants."
The researchers did not
look at the mutants that died from developmental disorders before
reaching maturity, despite being cared for by scientists.
Some of the insects had irregular legs. Others acquired complicated
patterns of colors on their wings. The segments of their antennae
took on different shapes.
Freed from natural
selection, flies have reveled in complexity.
Another objection Erwin and other critics have raised is that McShea
and Brandon's version of complexity does not jibe with how most
people define the term. After all, an eye does not just have many
different parts. Those parts also carry out a task together, and
each one has a particular job to do.
But McShea and Brandon
argue that the kind of complexity that they are examining could lead
to complexity of other sorts.
"The kind of
complexity that we're seeing in this Drosophila population is
the foundation for really interesting stuff that selection could
get hold of" to build complex structures that function to aid
survival, McShea says.
Molecular
Complexity
As a paleobiologist, McShea is accustomed to thinking about the kind
of complexity he can see in fossils - bones fitting together into a
skeleton, for example.
But in recent years a
number of molecular biologists have independently begun to think
much as he does about how complexity emerges.
In the 1990s a group of Canadian biologists started to ponder the
fact that mutations often have no effect on an organism at all.
These mutations are, in the jargon of evolutionary biology, neutral.
The scientists, including
Michael Gray of Dalhousie University in Halifax, proposed
that the mutations could give rise to complex structures without
going through a series of intermediates that are each selected for
their help in adapting an organism to its environment.
They dubbed this process
"constructive neutral evolution."
Gray has been encouraged by some recent studies that provide
compelling evidence for constructive neutral evolution. One of the
leaders in this research is Joe Thornton of the University of
Oregon.
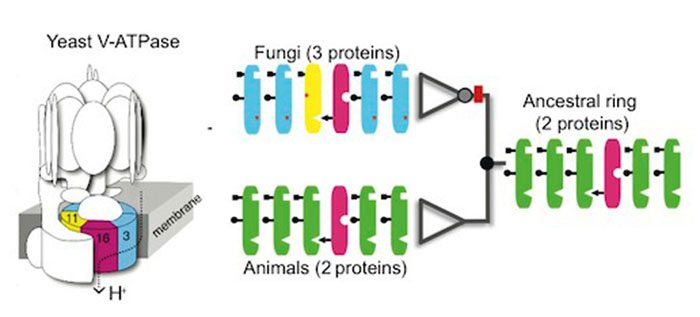
He and his colleagues
have found what appears to be an example in the cells of fungi.
In fungi, such as a
portobello mushroom, cells have to move atoms from one place to
another to stay alive. One of the ways they do so is with molecular
pumps called
vacuolar ATPase complexes.
A spinning ring of
proteins shuttles atoms from one side of a membrane in the fungus to
another. This ring is clearly a complex structure. It contains six
protein molecules.
Four of the molecules
consist of the protein known as Vma3. The fifth is Vma11 and the
sixth Vma16.
All three types of
protein are essential for the ring to spin.
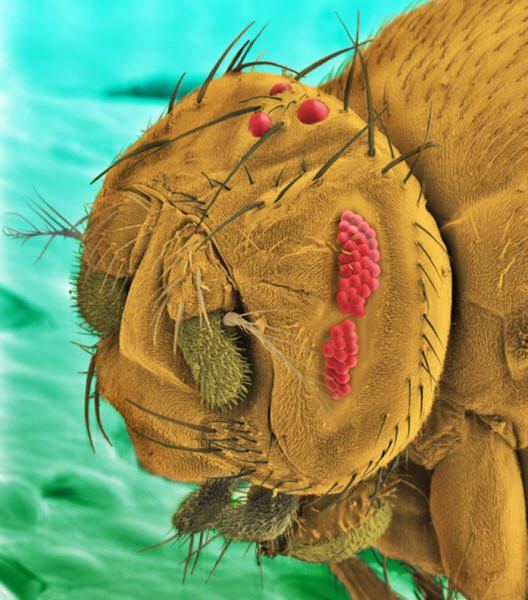
Scientists
have proposed that complexity can sometimes evolve
without the help of natural selection.
Here's an
example of how this might occur.
A: The
gene A encodes a protein with a structure that
allows eight copies of it to assemble into a ring.
B: The
gene accidentally duplicates. Initially, the two
kinds of proteins can combine in any order to
produce the same ring.
C:
Mutations take away some of the sites at which the
proteins can bind. Now they can only arrange
themselves in one particular combination.
The ring has
become more complex, but not because complexity was
favored by natural selection.
Illustration courtesy of Nature
To find out how this complex structure evolved, Thornton and his
colleagues compared the proteins with related versions in other
organisms, such as animals. (Fungi and animals share a common
ancestor that lived around a billion years ago.)
In animals, the vacuolar ATPase complexes also have spinning rings
made of six proteins.
But those rings are
different in one crucial way:
instead of having
three types of proteins in their rings, they have only two.
Each animal ring is made
up of five copies of Vma3 and one of Vma16. They have no Vma11.
By McShea and Brandon's
definition of complexity, fungi are more complex than animals - at
least when it comes to their vacuolar ATPase complexes.
The scientists looked closely at the genes encoding the ring
proteins. Vma11, the ring protein unique to fungi, turns out to be a
close relative of the Vma3 in both animals and fungi. The genes for
Vma3 and Vma11 must therefore share a common ancestry.
Thornton and his
colleagues concluded that early in the evolution of fungi, an
ancestral gene for ring proteins was accidentally duplicated. Those
two copies then evolved into Vma3 and Vma11.
By comparing the differences in the genes for Vma3 and Vma11,
Thornton and his colleagues reconstructed the ancestral gene from
which they both evolved. They then used that DNA sequence to create
a corresponding protein - in effect, resurrecting an
800-million-year-old protein.
The scientists called
this protein Anc.3-11 - short for ancestor of Vma3 and Vma11.
They wondered how the
protein ring functioned with this ancestral protein. To find out,
they inserted the gene for Anc.3-11 into the DNA of yeast. They also
shut down its descendant genes, Vma3 and Vma11.
Normally, shutting down
the genes for the Vma3 and Vma11 proteins would be fatal because the
yeast could no longer make their rings. But Thornton and his
co-workers found that the yeast could survive with Anc.3-11 instead.
It combined Anc.3-11 with
Vma16 to make fully functional rings.
University of
Oregon scientists have reconstructed the evolution of a
complex structure found in yeast and other fungi.
Left: Yeast
use a pump called vacuolar-ATPase to move charged
proteins across their membranes. One key part of this
pump is a ring made up of six interlocking proteins
(shown in color here).
Right: By
comparing the ring in fungi to the ring in animals, the
researchers have reconstructed its evolution. The
ancestral ring had two types of protein. The black
squares, triangles and circles show sites of the
proteins that can bind to other proteins at sites marked
with corresponding holes.
In fungi, one
of the genes duplicated, producing three types of
proteins. Some of the proteins lost sites where other
proteins could bind, marked here by red spots.
This
real-world example matches the scenario in the previous
figure.
Illustration courtesy of Nature
Experiments such as this one allowed the scientists to formulate a
hypothesis for how the fungal ring became more complex.
Fungi started out with
rings made from only two proteins - the same ones found in animals
like us. The proteins were versatile, able to bind to themselves or
to their partners, joining up to proteins either on their right or
on their left.
Later the gene for
Anc.3-11 duplicated into Vma3 and Vma11.
These new proteins kept
doing what the old ones had done:
they assembled into
rings for pumps.
But over millions of
generations of fungi, they began to mutate.
Some of those mutations
took away some of their versatility. Vma11, for example, lost the
ability to bind to Vma3 on its clockwise side. Vma3 lost the ability
to bind to Vma16 on its clockwise side.
These mutations did not
kill the yeast, because the proteins could still link together into
a ring. They were neutral mutations, in other words.
But now the ring had to
be more complex because it could form successfully only if all three
proteins were present and only if they arranged themselves in one
pattern.
Thornton and his colleagues have uncovered precisely the kind of
evolutionary episode predicted by the zero-force evolutionary law.
Over time, life produced more parts - that is, more ring proteins.
And then those extra parts began to diverge from one another.
The fungi ended up with a
more complex structure than their ancestors had. But it did not
happen the way Darwin had imagined, with 'natural selection'
favoring a series of intermediate forms.
Instead the fungal ring
degenerated its way into complexity.
Fixing
Mistakes
Gray has found another example of constructive neutral evolution (Evolutionary
origin of RNA editing) in the way many species edit their
genes.
When cells need to make a
given protein, they transcribe the DNA of its gene into
RNA, the single-stranded
counterpart of DNA, and then use special enzymes to replace certain
RNA building blocks (called
nucleotides) with other ones.
RNA editing is essential
to many species, including us - the unedited RNA molecules produce
proteins that do not work. But there is also something decidedly odd
about it.
Why don't we just have
genes with the correct original sequence, making RNA editing
unnecessary?
The scenario that Gray proposes for the evolution of RNA editing
goes like this:
an enzyme mutates so
that it can latch onto RNA and change certain nucleotides. This
enzyme does not harm the cell, nor does it help it - at least
not at first. Doing no harm, it persists. Later a harmful
mutation occurs in a gene.
Fortunately, the cell
already has the RNA-binding enzyme, which can compensate for this
mutation by editing the RNA.
It shields the cell from
the harm of the mutation, allowing the mutation to get passed down
to the next generation and spread throughout the population. The
evolution of this RNA-editing enzyme and the mutation it fixed was
not driven by natural selection, Gray argues. Instead this extra
layer of complexity evolved on its own - "neutrally."
Then, once it became
widespread, there was no way to get rid of it.
David Speijer, a biochemist at
the University of Amsterdam, thinks that Gray and his colleagues
have done biology a service with the idea of constructive neutral
evolution, especially by challenging the notion that all complexity
must be adaptive.
But Speijer worries they
may be pushing their argument too hard in some cases.
On one hand, he thinks
that the fungus pumps are a good example of constructive neutral
evolution.
"Everybody in their
right mind would totally agree with it," he says.
In other cases, such as
RNA editing, scientists should not, in his view, dismiss the
possibility that natural selection was at work, even if the
complexity seems useless.
Gray, McShea and Brandon acknowledge the important role of natural
selection in the rise of the complexity that surrounds us, from the
biochemistry that builds a feather to the photosynthetic factories
inside the leaves of trees.
Yet they hope their
research will coax other biologists to think beyond natural
selection and to see the possibility that random mutation can fuel
the evolution of complexity on its own.
"We don't dismiss
adaptation at all as part of that," Gray says. "We just don't
think it explains everything."
|