|

Associated Press
May 20, 2010
from
FoxNews Website
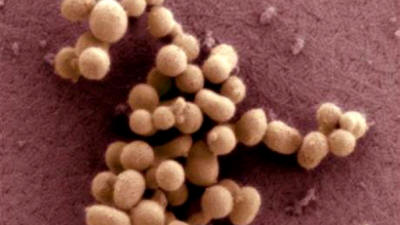
Craig Venter
Institute
A scanning electron micrograph image of
the synthetic
bacteria of M. mycoides JCVI-syn1.
WASHINGTON
Scientists announced a bold step
Thursday in the enduring quest to create artificial life. They've
produced a living cell powered by manmade DNA.
While such work can evoke images of Frankenstein-like scientific
tinkering, it also is exciting hopes that it could eventually lead
to new fuels, better ways to clean polluted water, faster vaccine
production and more.
Is it really an artificial life form?
The inventors call it the world's first synthetic cell, although
this initial step is more a re-creation of existing life - changing
one simple type of bacterium into another - than a
built-from-scratch kind.
But Maryland genome-mapping pioneer
J. Craig Venter said his
team's project paves the way for the ultimate, much harder goal:
designing organisms that work differently from the way nature
intended for a wide range of uses.
Already he's working with ExxonMobil in
hopes of turning algae into fuel.
"This is the first self-replicating
species we've had on the planet whose parent is a computer,"
Venter told reporters.
And the report, being published Friday
in the journal Science, is triggering excitement in this growing
field of synthetic biology.
"It's been a long time coming, and
it was worth the wait," said Dr. George Church, a Harvard
Medical School genetics professor. "It's a milestone that has
potential practical applications."
Scientists for years have moved single
genes and even large chunks of DNA from one species to another. At
his
J. Craig Venter Institute in Rockville, Md., and San Diego,
Venter's team aimed to go further.
A few years ago, the researchers
transplanted an entire natural genome - the genetic code - of one
bacterium into another and watched it take over, turning a goat germ
into a cattle germ.
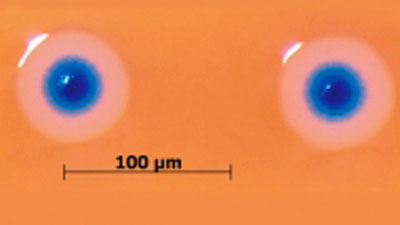
Scientists have
developed the first cell (shown here)
controlled by a
synthetic genome - a revolution some are calling artificial life.
Next, the researchers built from scratch another, smaller
bacterium's genome, using off-the-shelf laboratory-made DNA
fragments.
Friday's report combines those two achievements to test a big
question:
Could synthetic DNA really take over and drive a living
cell?
Somehow, it did.
"This is transforming life totally
from one species into another by changing the software," said
Venter, using a computer analogy to explain the DNA's role.
The researchers picked two species of a
simple germ named
Mycoplasma.
First, they chemically synthesized the
genome of M. mycoides, that goat germ, which with 1.1 million
"letters" of DNA was twice as large as the germ genome they'd
previously built.
Then they transplanted it into a living cell from a different
Mycoplasma species, albeit a fairly close cousin.
At first, nothing happened. The team scrambled to find out why,
creating a genetic version of a computer proofreading program to
spell-check the DNA fragments they'd pieced together.
They found that a typo in the genetic
code was rendering the manmade DNA inactive, delaying the project
three months to find and restore that bit.
"It shows you how accurate it has to
be, one letter out of a million," Venter said.
That fixed, the transplant worked.
The recipient cell started out with
synthetic DNA and its original cytoplasm, but the new genome "booted
up" that cell to start producing only proteins that normally would
be found in the copied goat germ.
The researchers had tagged the synthetic
DNA to be able to tell it apart, and checked as the modified cell
reproduced to confirm that new cells really looked and behaved like
M. mycoides.
"All elements in the cells after
some amount of time can be traced to this initial artificial
DNA. That's a great accomplishment," said biological engineer
Ron Weiss of the Massachusetts Institute of Technology.
Even while praising the accomplishment -
"biomolecular engineering of the highest order," declared
David Deamer of the University of California, Santa Cruz - many
specialists say the work hasn't yet crossed the line of truly
creating new life from scratch.
It's partially synthetic, some said, because Venter's team had to
stick the manmade genetic code inside a living cell from a related
species. That cell was more than just a container; it also contained
its own cytoplasm - the liquid part.
In other words, the synthetic part was "running on the 'hardware' of
the modern cell," University of Southern Denmark physics professor
Steen Rasmussen wrote in the journal Nature, which on Thursday
released essays of both praise and caution from eight leaders in the
field.
The environmental group
Friends of the Earth said the new work took
"genetic engineering to an extreme new level" and urged that Venter
stop until government regulations are put in place to protect
against these kind of engineered microbes escaping into the
environment.
Venter said he removed 14 genes thought to make the germ dangerous
to goats before doing the work, and had briefed government officials
about the work over the course of several years - acknowledging that
someone potentially could use this emerging field for harm instead
of good.
But MIT's Weiss said it would be far easier to use existing
technologies to make
bio-weapons:
"There's a big gap between science
fiction and what your imagination can do and the reality in
research labs."
Venter founded
Synthetic Genomics Inc.,
a privately held company that funded the work, and his research
institute has filed patents on it.
|


