|

November 04, 2010
from
NextBigFuture Website
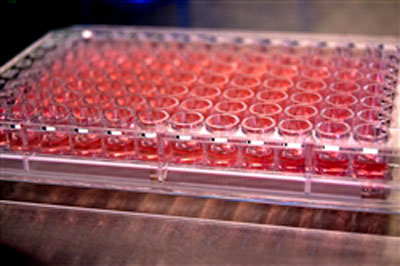
Each well in the
MIMIC system’s 96-well plastic plate represents a human immune
system.
The MIMIC system's
highly sensitive functional assays simulate a clinical trial
for a diverse
population without ever putting human subjects at risk.
Photo by Todd Lemoine,
courtesy of VaxDesign
A
DARPA effort, called Blue Angel,
has been working since May 2009 to develop a surge capacity for flu
viruses.
Eighteen months and $100 million later,
Blue Angel and the companies it funds have created new technologies
for developing, testing and quickly mass-producing new vaccines.
-
The DOD/Darpa progress on
tobacco plant production (with several companies) of vaccine
is progressing to industrialization to ramp up production of
a seed vaccine instead of using chicken eggs.
-
Speeding phase 3 testing and
lowering costs - Now, pharmaceutical company with a
candidate vaccine needs to enroll 10,000 people for three
years and $100 million. An alternative may be
MIMIC, a DARPA technology
developed by Florida-based biotechnology company VaxDesign
Corp. $1 million and 2 months to predicts the vaccine to
produce for humans.
-
Venter working on synthetic
biology for 12 hour identification and production of seed
vaccine.
-
DNA vaccine technology also
being developed in separate projects. Success could scale up
the seed vaccine in 12 hours to fully produce the vaccine
doses in one day.
For the largest program, called AMP for
Accelerated Manufacture of Pharmaceuticals, companies in four states
are building facilities where they can quickly produce vaccine-grade
proteins grown in the cells of tobacco plants.
Once they produce the proteins, the goal
is for each company to scale up its process to produce 100 million
doses of H1N1 flu vaccine per month. Existing vaccine manufacturers
worldwide produce a fraction of that - about 300 million doses of
vaccine in six months, Magill said.
Craig Venter
had indicated that synthetic
biology can produce the seed stock for a vaccine in 12 hours. It
would need to be used in conjunction with DNA vaccine technology to
achieve one day vaccine production.
Gutmann asked Venter whether, by next flu season, we could,
"have a one-day production, through
synthetic biology, of a flu vaccine?"
To which he answered that researchers
could produce the seed stock for the vaccine in just 12 hours.
Venter added that with,
"rapid DNA sequencing, we can
predict, we think, well in advance what the changes will be for
next year's flu before the WHO even makes the decision as to the
vaccine stocks."
Production, he said, is a whole
different story entirely.
Prather agreed.
"If you're still making [a vaccine]
in chicken eggs, it's not going to happen in a day. It's just
not going to happen," she said.
"So, there's a difference between
the tools of synthetic biology being able to give you what that
starting material is, if we're stuck with chicken eggs it's not
going to happen, if you go to chicken cell culture, it's going
to be faster, if the DNA vaccine technology proves out and you
can do it in microbes, you can absolutely do it in a day."
This, she was quick to point out, is an
immunological issue, not a synthetic biology problem.
Vaccines are produced in steps, beginning with getting a sample of
the active virus. From the original virus, “seeds” are used to grow
the virus in hundreds of millions of chicken eggs - a time-consuming
process developed more than 50 years ago. After the virus particles
are grown, they’re purified to make vaccine.
AMP set out to speed up the process by looking at a range of animals
and plants whose cells could produce high-quality proteins that
would work well in people, Magill said.
What emerged from the first round of
experiments were tobacco plants.
“Think about walking through the
woods on a rainy day. You walk through on Tuesday and there’s
nothing there, and you take the same walk on Wednesday and
suddenly there’s a mushroom that’s a foot high and it grew
overnight,” Magill said.
“Anything in nature that produces a
tremendous amplification of biomass was of interest,” he added.
“Clearly these weeds - that’s really what tobacco plants are -
grow very fast, and that’s what we captured.”
Plants with the fastest-growing cells
will be able to produce more proteins in a shorter time for
vaccines, he explained.
Four companies are working to transform protein-producing tobacco
plants from a proof of concept to a demonstration of the capability.
The next step will be to develop full industrial processes for
producing the proteins.
The companies are,
-
Fraunhofer USA - Center for
Molecular Biotechnology in Delaware, Kentucky
-
Bioprocessing in Owensboro
-
A consortium called Project
GreenVax, whose partners are,
-
Texas A&M University system
-
a Texas company called G-Con
-
Medicago USA in North
Carolina
“They’re all using tobacco plants,
and there’s a little variation on the theme,” Magill said.
“But the approaches - what do you
put in the plants, how do you infect the plant cells, what kind
of vectors [carriers] do you use, what is the nature of the
protein, how is it purified - all of these are actually quite
different.”
The companies all are making progress,
he said.
One of them, Fraunhofer, already has a
product in Phase 1 clinical trials - the first stage of testing in
people.
“The final trial will go on for six
months, because we have to do safety monitoring,” Magill said.
“But we’ll know whether the technology worked probably about the
end of January.”
Another Blue Angel project is a
technology called Modular Immune In Vitro Constructs, or
MIMIC, which Magill calls “an
immune system in a test tube.” DARPA created MIMIC to quickly test
new vaccines for safety and effectiveness.
Pharmaceutical companies that produce candidate vaccines initially
don’t actually know if the drug will improve a person’s immunity or
will be safe when administered. That’s why in the United States the
Food and Drug Administration (FDA) requires companies to hold a series of
clinical trials before drugs are approved for market.
As a pharmaceutical company with a candidate vaccine, Magill said,
“all I can do is commit to a Phase 3
[effectiveness] study in which I will have to enroll 10,000
people over the course of about three years in order to… show
that my new vaccine in this case would be as good as the
traditional egg-based vaccine.
“So 10,000 people, three years, $100
million,” he said.
An alternative may be MIMIC, a DARPA
technology developed by Florida-based biotechnology company
VaxDesign Corp.
Each of MIMIC’s 128-by-85-millimeter plastic plates contains 96 tiny
wells filled with mixtures of human immune cells and biological
molecules. Each well represents a human immune system.
The system can predict the effectiveness of vaccine additives called
adjuvants and molecules that the immune system recognizes called
antigens, VaxDesign officials said, adding that it
can,
-
predict dosing, dose timing
and cross-protection against other viral strains
-
determine the potency of
stockpiled vaccines
-
compare the effects of different
manufacturing methods on vaccine potency
“It’s a very clever technology,”
Magill said. “I can look at the immune responses in the MIMIC
system and tell you that this is going to work, this is going to
protect patients, they’re not going to get sick and it’s going
to be really safe.”
In September, Sanofi Pasteur, the
vaccine division of the Lyon, France-based Sanofi-Aventis Group,
signed a binding agreement to buy VaxDesign for $60 million.
The full potential of MIMIC - to take the place of clinical trials -
could take years to realize.
But Magill said he has confidence in
the technology.
“Where this will be useful is in
what we call the down-select - when you’re in the business and
you’ve got five vaccine candidates and you’re not sure which one
is going to work,” he said.
Today, to down-select the best
candidate a company would have to do a year-long Phase 1 study
for each candidate that would cost $5 million to $7 million per
trial.
“But what if I can just replace all
that by going into MIMIC up front?” Magill said.
“Let’s say I spend $1 million in MIMIC, but I get the answer in
two months and that predicts the vaccine that I need to take
into humans,” he said.
“That’s huge. And I think the
likelihood of that occurring is pretty high.”
MIMIC will work in parallel with AMP to
test candidate H1N1 vaccines, Magill said, and both will complement
other projects that also are part of Blue Angel.
Technologies developed for Blue Angel eventually will apply to a
range of flu viruses and other diseases, Magill added.
“Blue Angel’s vaccine portfolio
alone has generated four facilities, four [technical]
approaches, two clinical trials, two [FDA investigational new
drug applications], the MIMIC and a variety of other spinoff
technologies,” he said, adding that it could take a decade to
commercialize the technology.
"Such an outcome for plant-based vaccines would be amazing," he
said.
“We don’t see very often that a response like this essentially
creates a new industry. But we’ll see,” Magill said.
“You still have to go through
clinical trials… and work through all the issues. But I would
say initially things are quite pleasing and somewhat promising.”
|

