|

by Akyildiz, Pierobon, et.al.
March 2015
from
Academia Website
PDF Version

First, there was the Internet of Things (IoT), then the
Internet of Bodies (IoB), the Internet of Everything (IoE),
and finally,
Big Pharma and the military are going into
your blood to construct the Internet of Bio-NanoThings (IoBNT).
You
might have hoped for the Internet of Nothing, but
instead, you are getting the Internet of Universal Skynet (IoUS).
This paper from March 2015 is a primer that anybody can
understand, including you.
The
IoBNT is the final building block of the surveillance
network, bridging all living things from the biochemical
domain into the electrical domain of the Internet.
There was no warning that nanotechnology of this sort
was being pumped into your veins when you received a
mRNA injectable from Pfizer or Moderna.
Not
a word from the government, Big Pharma, or the Military.
There was no Informed Consent offered.
The
non-stop propaganda blared "Safe and Effective"...
Technocracy is literally on track to conquer the human
race, while humans don't have a clue that a war is being
waged against them in the first place.
This
is the topic of
OMNIWAR: A SYMPOSIUM on
September 21.
I will be participating with Catherine
Austin Fitts, David Hughes, Daniel Broudy and Lissa
Johnson.
It
is free but you should RSVP to get further notices. This
is the first global livestream that will reach all
corners of the world.
Can
you see why I am asking to shout it from the roof top?
Patrick Wood
The IoBNT stands as a paradigm-shifting concept for communication
and network engineering.
Novel challenges are faced to develop
efficient and safe techniques for the exchange of information,
interaction, and networking within the biochemical domain while
enabling an interface to the electrical domain of the Internet.
Abstract
The Internet of Things (IoT) has become an important research topic
in the last decade, where things refer to interconnected machines
and objects with embedded computing capabilities employed to extend
the Internet to many application domains.
While research and development continue for
general IoT devices, there are many application domains where very
tiny, concealable, and non-intrusive Things are needed.
The properties of recently studied nanomaterials, such as
graphene,
have inspired the concept of Internet of NanoThings (IoNT), based on
the interconnection of nanoscale devices.
Despite being an enabler for many applications,
the artificial nature of IoNT devices can be detrimental where the
deployment of NanoThings could result in unwanted effects on health
or pollution.
The novel paradigm of the Internet of Bio-Nano
Things (IoBNT) is introduced in this paper by stemming from
synthetic biology and nanotechnology tools that allow the
engineering of biological embedded computing devices.
Based on biological cells, and their functionalities in the
biochemical domain, Bio-NanoThings promise to enable applications
such as intra-body sensing and actuation networks, and environmental
control of toxic agents and pollution.
The IoBNT stands as a paradigm-shifting concept
for communication and network engineering, where novel challenges
are faced to develop efficient and safe techniques for the exchange
of information, interaction, and networking within the biochemical
domain, while enabling an interface to the electrical domain of the
Internet.
Introduction
The Internet of Things (IoT) defines a cyber physical paradigm,
where all types of real-world physical elements (sensors, actuators,
personal electronic devices, or home appliances, among others) are
connected, and are able to autonomously interact with each other.
This new form of seamless connectivity is the
enabler for many applications such as machine to machine
communication, real time monitoring of industrial processes, smart
cities, smart grids for energy management, intelligent
transportation, environmental monitoring, infrastructure management,
medical and healthcare systems, building and home automation, and
large scale deployments.
The Internet of Things became a focus for
research and development in the last 15 years. A large amount of
investments for Internet of Things was and is still being made by
government agencies and industry worldwide.
Recently, the concept of IoT has been revised in light of novel
research advances made in the field of nanotechnology and
communication engineering, which enable the development of networks
of embedded computing devices, based on nanomaterials such as
graphene or metamaterials, having scales ranging from one to a few
hundred nanometers, called nanothings.
The Internet of NanoThings (IoNT), introduced for
the first time in, 1 is proposed as the basis of numerous
future applications, such as in the military, healthcare, and
security fields, where the nanothings, thanks to their limited size,
can be easily concealed, implanted, and scattered in the
environment, where they can cooperatively perform sensing,
actuation, processing, and networking.
While nanothings can push the engineering of devices and systems to
unprecedented environments and scales, similarly to other devices,
they have an artificial nature, since they are based on synthesized
materials, electronic circuits, and interact through electromagnetic
(EM) communications. 1
These characteristics can be detrimental for some
application environments, such as inside the body or in natural
ecosystems, where the deployment of nanothings and their EM
radiation could result in unwanted effects on health or pollution.
A novel research direction in the engineering of nanoscale devices
and systems is being pursued in the field of biology, by combining
nanotechnology with tools from synthetic biology to control, reuse,
modify, and reengineer biological cells. 2
By stemming from an analogy between a biological
cell and a typical IoT embedded computing device, a cell can be
effectively utilized as a substrate to realize a so-called Bio-NanoThing,
through the control, reuse, and reengineering of biological cells'
functionalities, such as sensing, actuation, processing, and
communication.
Since cells are based on biological molecules and biochemical
reactions, rather than electronics, the concept of Internet of
Bio-NanoThing (IoBNT), introduced in this article, is
expected to be paradigm shifting for many related disciplines, such
as communication and network engineering, which is the focus of this
article.
The execution of DNA-based instructions, the
biochemical processing of data, the transformation of chemical
energy, and the exchange of information through the transmission and
reception of molecules, termed molecular communication (MC), 3
are at the basis of a plethora of applications that will be enabled
by the IoBNT, such as:
-
Intra-body sensing and actuation, where
Bio-NanoThings inside the human body would collaboratively
collect health-related information, transmit it to an
external healthcare provider through the Internet, and
execute commands from the same provider such as synthesis
and release of drugs.
-
Intra-body connectivity control, where
Bio- NanoThings would repair or prevent fail-ures in the
communications between our internal organs, such as those
based on the endocrine and the nervous systems, which are at
the basis of many diseases.
-
Environmental control and cleaning, where
Bio-NanoThings deployed in the environment, such as a
natural ecosystem, would check for toxic and pollutant
agents, and collaboratively transform these agents through
bioremediation, e.g. bacteria employed to clean oil spills.
This article is organized as follows.
-
First, Bio- Nano-Things are defined in
light of the tools available today from synthetic biology
and nanotechnology.
-
Second, the application of communication
engineering to design Bio-NanoThings telecommunications is
detailed, while the challenges to engineer Bio-NanoThings
networks and Internet connections are discussed.
-
Third, we describe further research
challenges for the realization of IoBNT.
-
Finally, we conclude the article.
Bio-NanoThings
Within the scope of the IoBNT, Bio-NanoThings are defined as
uniquely identifiable basic structural and functional units that
operate and interact within the biological environment.
Stemming from biological cells, and enabled by
synthetic biology and nanotechnology, Bio-NanoThings are expected to
perform tasks and functionalities typical of the embedded computing
devices in the IoT, such as sensing, processing, actuation, and
interaction with each other.
BIOLOGICAL
CELLS AS THE SUBSTRATES OF BIO-NANOTHINGS
A biological cell is the basic unit of life, consisting of a
membrane that encloses a mixture of highly specialized
molecules, with defined chemical composition and function, which
may also be organized into functional structures [4].
A mapping between the components of a typical
IoT embedded computing device, and the elements of a cell,
becomes apparent if we compare electrons' propagation in
semiconductors to functionally similar, although much more
complex, biochemical reactions.
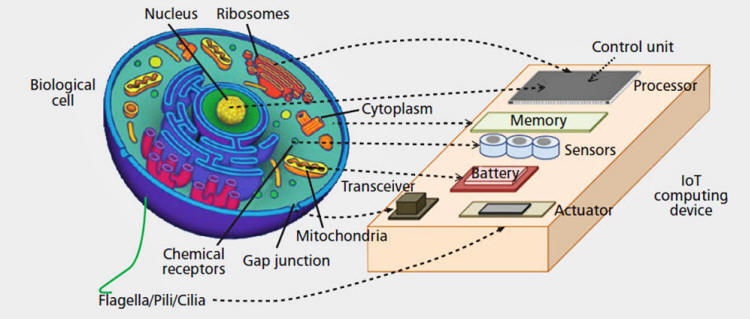
In this context, as illustrated in Fig. 1
above,
some examples are as follows.
The control unit, which contains the embedded software of the
device, would correspond to the genetic instructions densely
packed into the cells' DNA molecules, which encode protein
structures, the cell's "data units," and regulatory sequences,
similar to the software conditional expressions.
The memory unit, which contains the values of the embedded
system data, would correspond to the chemical content of the
cytoplasm, i.e. the interior of the cell, comprised of molecules
synthesized by the cell as a result of DNA instructions, and
other molecules or structures, e.g. vesicles, exchanged with the
external environment.
The processing unit, which executes the software instructions
and manages memory and peripherals, would correspond to the
molecular machinery that, from the DNA molecules, through the
so-called transcription and translation, generates protein
molecules with instruction-dependent types and concentrations.
The power unit, which supplies the energy to maintain the
electrical currents in the embedded system's circuits, would
correspond to the reservoir in the cell of the Adenosine
TriPhosphate (ATP) molecule, which is synthesized by the cell
from energy supplied from the external environment in various
forms, and provides the energy necessary for the cell's
biochemical reactions to take place.
The transceivers, which allow the embedded systems to exchange
information, would correspond to the specific chains of chemical
reactions, i.e. signaling pathways, through which cells exchange
information-bearing molecules.
Sensing and actuation, which allow embedded systems to acquire
data and interact with the environment, would correspond to the
capability of a cell to chemically recognize external molecules
or physical stimuli, e.g. light or mechanical stress, and to
change the chemical characteristics of the environment or
mechanically interact through moving elements, such as flagella,
pili, or cilia.
Enabling Technologies
and Challenges
The discipline of synthetic biology is providing tools to control,
reuse, modify, and reengineer the cells' structure and function, and
it is expected to enable engineers to effectively use the biological
cells as programmable substrates to realize Bio-NanoThings as
biological embedded computing devices. 2
DNA sequencing and synthesis technologies,
enabling the reading and writing of genetic code information in the
DNA molecules of biological cells, are giving engineers an
increasingly open access to the set of structural and functional
instructions at the basis of life.
In particular, the engineering of synthetic biological circuits
5 through genetic code manipulation has enabled the
programming of specifically designed functions to be executed by
cells.
A biological circuit is a set of genes that
encode proteins and regulatory sequences, which link together the
protein synthesis by mechanisms of mutual activation and repression.
The functions today successfully developed via
biological circuits range from AND and OR logic gates, to various
types of tunable oscillators, toggle switches, and counters.
The development of databases with characterized
standard biological circuit parts with known functions and
behaviors, e.g. BioBricks, and tools to combine them into more
complex designs, 6 are pushing synthetic biology to a
future development similar to that experienced by integrated
electrical circuit design in electronics.
As a consequence, engineers will be soon able to
gain full access to the functionalities of the aforementioned cells'
elements, and reuse cells and their features, without requiring an
in-depth knowledge of biotechnology.
One of the latest frontiers in synthetic biology
is the development of artificial cells, enabled, among others, by
tools from nanotechnology.
Artificial cells have minimal functionalities and structural
components compared to natural cells, and are assembled bottom-up by
encapsulating the necessary elements into either biological or fully
synthetic enclosing membranes. 7
Artificial cells can therefore contain genetic
information, the related molecular machineries for their
transcription, translation, and replication, and all the required
specialized molecules and structures.
Artificial cells are expected to enable a more
agile and controllable use of synthetic biological circuits by
removing all the additional complexity of natural cells that are not
necessary to perform the designed functions.
Although still in its infancy, this technology has been successfully
applied, e.g. for drug delivery, gene therapy, and artificial blood
cell production, and it is expected to deliver ideal substrates for
synthetic biology with a more predictable behavior.
Although very promising, the aforementioned
technologies have to provide solutions to major research challenges
in biotechnology and engineering before being considered as reliable
tools for the realization of Bio-NanoThings.
Focusing on the engineering design viewpoint, a
major challenge is to develop reliable mathematical and physical
models, and computer simulation environments, able to capture the
peculiar characteristics of the biological processes underlying
engineered cells, such as intrinsic non-linear phenomena and
processes with noisy outcomes.
Moreover, engineered cells, similar to natural
cells, reproduce and mutate, i.e. tend to randomly change parts of
their genetic programs, and selectively evolve, i.e. tend to
maintain the best mutations for their survival while reproducing,
adding possible problems but also new degrees of freedom to the
biological device designer.
Another challenge that needs to be considered is related to
bioethics and security, since autonomously evolving engineered
organisms could pose a threat to the natural ecosystems, and even
become new pathogens.
The recent development of "kill" switches into
biological circuits, able to stop cell reproduction or trigger cell
destruction upon an external command, is only partially addressing
these problems.
Bio-NanoThings Communications
At the basis of the IoBNT concept there is the need for Bio-NanoThings
to communicate with each other, and interact on the basis of the
exchanged information.
Since Bio-NanoThings stem from the engineering of
biological cells, as detailed above, the natural environment is the
main inspiration for studying communication techniques for IoBNT.
MOLECULAR
COMMUNICATION IN NATURE
In nature, the exchange of information between cells is based on
the synthesis, transformation, emission, propagation, and
reception of molecules through biochemical and physical
processes.
This information exchange, recently
classified in telecommunications engineering as MC, 1
enables cells' interactions and coordination of uni-cellular and
multi-cellular organisms, populations, and multi-species
consortia, and participates in most of the major cellular
functionalities such as cell growth and proliferation.
MC in cells is based on the aforementioned signaling pathways,
which are chains of chemical reactions that process information
signals modulated into chemical characteristics, such as
molecule concentration, type, and energy state, and propagate
them from a source, or transmitter, to a destination or
receiver. 4
Cell signaling pathways can be classified on
the basis of the distance between source and destination into
intracrine (source and destination are within the same cell),
juxtracrine (source and destination are cells in contact with
each other), paracrine (source and destination are in the
vicinity of each other, but not in contact), or endocrine
(source and destination are distant from each other).
An example of intracrine communication is given by the
intracellular transport of molecules or molecule structures
operated by cytoskeletal molecular motors.
Molecular motors are intracellular
specialized proteins able to convert the aforementioned ATP
molecules into mechanical energy.
The cytoskeletal molecular motors are able to
bind to a particular cargo, such as vesicles enclosing sets of
molecules, or whole cell organelles, attach to the microfilament
structures that compose the cell's skeleton, and crawl along
them transporting the cargo from the nucleus to the membrane of
the cell and vice versa.
The exchange of molecules, such as calcium ions Ca2+, between
two cells connected by communicating junctions in their
membrane, is an example of juxtacrine communication.
Several examples in nature, such as the
signaling during a cardiac contraction happening between
muscular cells, or myocytes, show how a small quantity of
molecules can flow by diffusion between neighboring cells, and
be responsible for synchronizing coordinated actions.
Bacteria show several means of communication in nature, such as
the paracrine communication underlying the emission of signaling
molecules called autoinducers by members of a population.
In this process, called bacterial quorum
sensing, the autoinducers diffuse within the intercellular space
and, upon reception, allow the bacteria to estimate the
population density, and have a correlated response, such as the
production of specific types of proteins.
Bacteria can also exchange specific DNA
molecules, i.e. plasmids, via direct contact, through a process
called conjugation, and carry the plasmids to other distant
bacteria within the intercellular space by swimming according to
chemical trails, with a process called chemotaxis.
In multicellular organisms, an example of endocrine
communication is realized through signaling molecules called
hormones that are emitted from cells composing glands, propagate
through the circulatory system, and are received by the cells of
distant organs, where they elicit specific responses, such as
increased cell growth and reproduction.
CHALLENGES
IN ENGINEERING MOLECULAR COMMUNICATION FOR IOBNT
Within the IoBNT, Bio-NanoThings are expected to interact with
each other by exchanging various types of information, e.g.
synchronization signals, values of sensed chemical/physical
parameters, results of logical operations, and sets of
instructions and commands.
The engineering of the communication
techniques to support these interactions in the biological
environment has to stem from solutions found in nature, such as
those described above.
One of the major challenges is to understand how these natural
solutions can be controlled, modified, or reengineered for the
transmission of information that can be different from the
natural.
By stemming from the aforementioned tools
that are being developed in synthetic biology and
nanotechnology, engineers have recently started to analyze
several different possibilities to realize MC systems, either by
genetically reprogramming cells' behaviors within their natural
communications, 8 or by developing totally new
artificial communication systems by assembling natural
biological components. 9
Examples of MC systems that have been envisioned so far can be
classified on the basis of the distance range that they are
expected to cover from transmission to reception.
For example, the control of juxtracrine
communications through the genetic programming of biological
cells can enable the engineering of networks where Bio-
NanoThings are in contact with each other, e.g. when organized
in a tissue or biofilm. 10
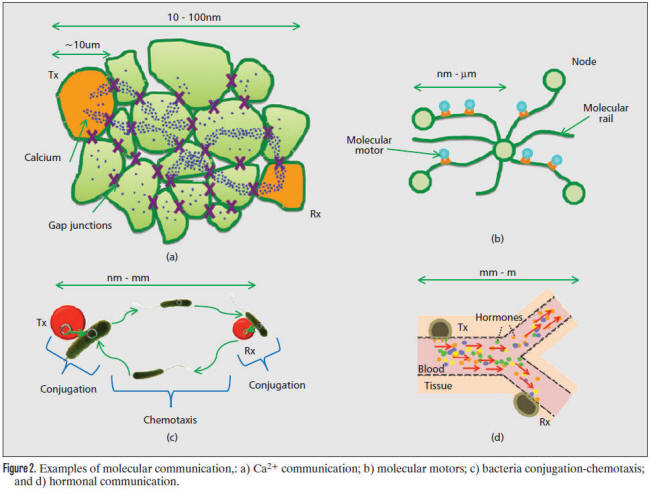
This MC technique, usually referred to the
aforementioned Ca2+ exchange, shown in Fig. 2a, covers distances
proportional to the thickness of cell membranes, and it can be
considered as very short range (tens to hundreds of nm) MC.
The aforementioned cytoskeletal molecular
motors can be considered for the realization of MC in the short
range (nm-mm), 11 as shown in Fig. 2b, to cover
intracrine Bio-NanoThings communications.
Communication engineers have also combined models of the
bacterial conjugation and chemotaxis processes described above
to theoretically study a possible artificial MC system, which
can be considered, according to the known chemotaxis
characteristics, as covering the medium range (mm-mm). 9
In particular, the information is represented
into DNA molecules, i.e. plasmids, which are loaded at the
transmitter location into bacteria and extracted from the same
bacteria at the receiver through a conjugation process.
These bacteria are able to swim by chemotaxis toward the
receiver, by following the receiver's release of specific
molecules, i.e. chemoattractants, as shown in Fig. 2c.
An example of long range (mm-m) MC system has
been envisioned by stemming from the hormonal communication
within the human endocrine system, 10 as shown in
Fig. 2d.
From the telecommunications engineering
perspective, one of the main challenges stands in mapping MC
into the classical elements of an engineered communication
system, and in the use of tools from systems and information
theory with the final goals of modeling and analyzing the main
telecommunication characteristics and performance, such as
range, delay (latency), capacity, throughput, and bit error
rate. 13
The knowledge of these characteristics will then allow for the
comparison and classification of possible different techniques
to realize MC for different IoBNT application scenarios, and the
optimization of their design and realization.
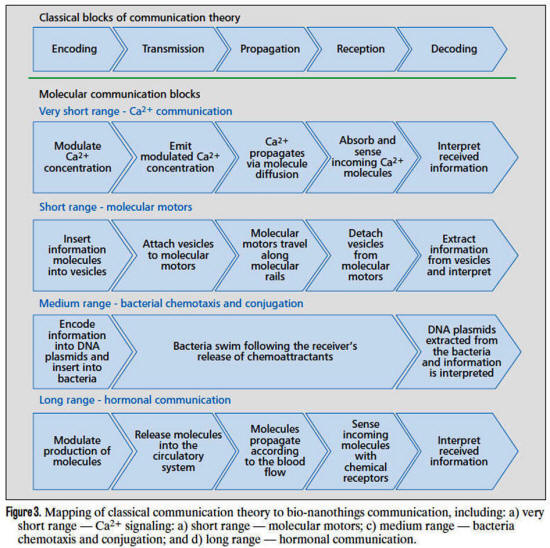
Examples of the aforementioned mapping are
shown in Fig. 3, where the main processes involved in each MC
system described above are divided into communication elements
as follows.
Encoding and decoding are related to how the
information to be transmitted is represented into one or more
molecule characteristics, such as sets of particular molecule
types and numbers (molecular motors and hormonal communication),
composition of biological macromolecules, such as DNA plasmids
(bacteria conjugationchemotaxis), or released molecule
concentration (Ca2+ exchange).
Transmission and reception involve the chemical and physical
processes to initiate the propagation of molecules, e.g. the
encapsulation into vesicles for molecular motor transportation,
the release of molecules in a fluid, such as the blood stream,
or through a junction between two adjacent cells, or the release
of bacteria upon the presence of chemoattractant molecules in
the environment.
Finally, the propagation is concerned with the mobilization of
the information-bearing molecules from the location of the
transmitter to the receiver, such as through molecular motor
crawling along microfilament structures, diffusion through
membrane junctions, diffusion and advection in the blood stream,
and bacterial chemotaxis toward the chemoattractant source
(receiver).
While a great amount of the literature within the MC field has
been devoted to the modeling and analysis of the aforementioned
systems through simplifying assumptions, which increase the
mathematical tractability of the underlying physical and
chemical phenomena, there is still a long way to go for a
communication engineer to fully understand how to design
realistic MC systems for IoBNT communications.
The main challenges are given by the conversion of these
simplified models to more realistic scenarios.
For example, the free diffusion models
considered so far in MC engineering for the propagation and
reaction of molecules in the intracellular environment, e.g. in
Ca2+ communication, have to be revised to include more realistic
phenomena, such as the effect of high concentrations of
macromolecules, e.g. proteins, called macromolecular crowding.
Another example is given by the endocrine
propagation, so far considered for a small subset of
well-defined blood vessels, where models should take into
account not only the whole average physiology of the human
cardiovascular system, but also that the specific
characteristics of each individual can result in very different
propagation dynamics.
Also, the models of bacteria chemotaxis used so far in MC
engineering are only based on the behavior and properties of
single bacteria and in-vitro environments, where in fact more
realistic environments, such as within the human body, and the
fact that bacteria can replicate and proliferate dynamically and
interact within multispecies consortia, should be taken into
account.
Other challenges for the development of
reliable analytical tools for MC engineering are given by the
non-linear nature of many biochemical phenomena, and the
presence of very different noise sources, such as genetic
mutations, compared to classical systems.
Bio-Nanothing Networks and The
Internet
Within the IoBNT, Bio-NanoThings are expected to not only
communicate with each other, but also interact into networks, which
will ultimately interface with the Internet.
To this end, the definition of network
architectures and protocols on top of the aforementioned MC systems
is an essential step for IoBNT development.
A further challenge for the IoBNT is the
interconnection of heterogeneous networks, i.e. composed of
different types of Bio-NanoThings and based on different MC systems.
Finally, the realization of interfaces between the electrical domain
of the Internet and the biochemical domain of the IoBNT networks
will be the ultimate frontier to create a seamless interconnection
between today's cyber-world and the biological environment.
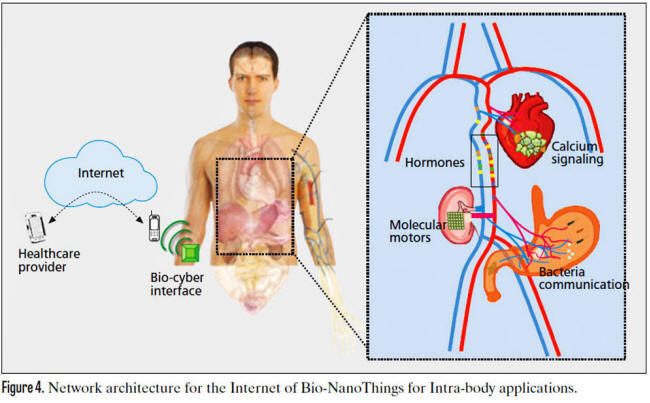
In Fig. 4 we show a possible scenario where a
complete IoBNT, composed of several networks based on different MC
systems, is deployed inside the human body, and interfaces through a
personal electrical device connected to the Internet to deliver
intra-body status parameters (and receive commands and instructions)
to (from) a "healthcare" provider.
CHALLENGES
FOR REALIZING BIO-NANOTHING NETWORKS
While the engineering of computer networks is a well-established
field, where several different solutions have been provided for
many different technologies and application scenarios, the
design of networks within the biological environment, and based
on the MC paradigm as the physical medium, poses new challenges
to the networking community.
For example, molecular information generally
does not follow predictable and definite propagation directions,
as otherwise done by electromagnetic signals in classical
communications. 13
The diffusion of molecules, the bacterial chemotaxis, and the
filaments supporting molecular motors, tend to cover random
patterns between source and destination.
This and other peculiarities, such as the
non-linear nature of many biochemical phenomena, make it
particularly challenging to utilize classical techniques for
regulating Bio-NanoThings access to shared media, such as
fluids, addressing Bio-NanoThings, and designing information
routing mechanisms, which are important basic aspects of
computer networks.
As done for the MC systems, one possible solution will be to
model, analyze, and reuse the mechanisms of interactions of
multiple cells in nature, such as in bacteria populations
14 and multispecies consortia, or within the tissues of
multi-cellular organisms, to relay the IoBNT information.
In this direction, a solution for the interconnection of
heterogeneous Bio-NanoThing networks, based on different MC
systems, might as well come from the natural way our body
manages and fuses several types of information to maintain a
stable, healthy status, or homeostasis. 4
These intra-body processes allow
heterogeneous communications to occur at various scales,
translating from intracrine communications within a cell, to
juxtacrine communication within tissues, to endocrine
communications between different organs.
For example, the cells of the pituitary gland
perform this type of translation by releasing hormones to body
organs to control several processes, such as growth, blood
pressure, temperature, and sleeping patterns, as a result of the
reception of other hormones from the cells of the adjacent
hypothalamic tissue.
Biological circuits based on these processes could effectively
provide a set of genetic instructions that mimic the classical
gateways between different subnets on the Internet.
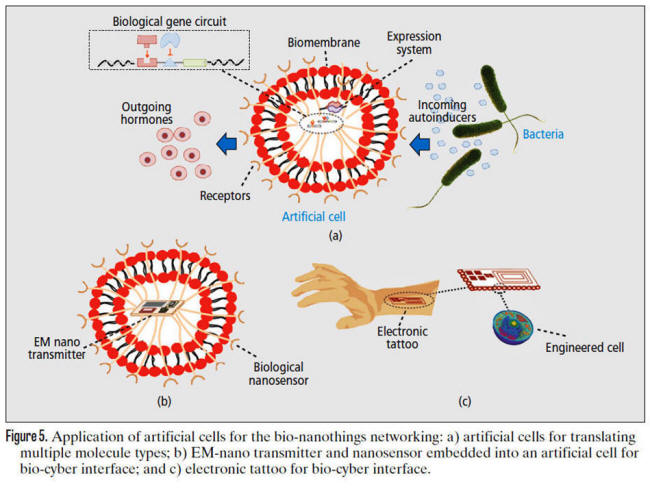
Figure 5a illustrates a general example of an
artificial cell that translates the information encoded into
molecules emitted from engineered bacteria into hormones that
can be secreted into the circulatory system.
In this design, receptors would intercept the incoming molecules
that, through a cascade of chemical reactions, would activate a
biological circuit, which in turn would synthesize proteins able
to trigger the necessary chemical reactions to produce the
hormones.
CHALLENGES FOR BIO-CYBER
INTERFACES
A bio-cyber interface is here defined as the set of processes
necessary to translate information from the biochemical domain
of Bio-NanoThing networks to the Internet cyber-domain, which is
based on electrical circuits and electromagnetic communications,
and vice versa.
One of the main challenges for the
realization of these interfaces stands in the engineering of
chemical and physical processes able to accurately read the
molecule characteristics where information is encoded, and
translate them into the modulation of electromagnetic
parameters.
A possible solution in this direction might
come from novel chemical and biological sensors enabled by
nanotechnology, which promise unprecedented sensing
capabilities. 15
These sensors are in general composed of materials characterized
by electrical or electromagnetic properties that can be altered
by the presence of determinate molecules or molecule complexes,
such as biological receptors bound to molecules, and accordingly
modulate the current in an electrical circuit.
The major problems for using this sensing
technology for IoBNT applications stand in their currently high
latency, low selectivity, lack of standardized response, and,
more importantly, unknown biocompatibility, which is considered
next.
Biocompatibility, intended here as the
property of an engineered system of limiting its action on the
biological environment exclusively to its intended function,
without any unwanted alteration of biological parameters, is
another challenge for the deployment of bio-cyber interfaces,
especially for intra-body IoBNT applications as shown in Fig. 4.
Given the limited size of the aforementioned
nanosensors, and current promising research results in
electromagnetic (EM) nanocommunications, we envision the
possibility to develop bio-cyber interfaces by encapsulating
biological nanosensors and EM nanocommunication units within the
aforementioned artificial cells, as shown in Fig. 5b.
In this design, the biological nanosensor would be responsible
for interfacing chemical and electrical domains, the EM nano-communication
unit would wirelessly communicate with electrical devices
outside of the biological environment, and the artificial cell
would assure biocompatibility.
However, a challenge lies in the ability to
produce sufficient power for the wireless transmitter to emit
electromagnetic waves that can propagate through the artificial
cell membrane.
At the same time, approaches are also required to harvest energy
for the transmitter unit from within the cell.
Another alternative is to push the
electrical/EM domain at the physical interface between the
biological environment and the external world, such as the skin
for intra-body IoBNT applications.
In this direction, electronic tattoos,
similar to those based on radio frequency identification
(RFID) technology, which allow users to authenticate devices
within close range, could incorporate a bio-cyber interface able
to sense bio-chemical information from cells on the epidermis,
sweat glands, or nervous terminations, and communicate it
wirelessly to nearby external electronic devices.
Further Challenges
We now briefly mention some further challenges to be faced for the
IoBNT development.
The IoBNT enabling technologies discussed in this
article could pose serious security threats if handled with
malicious intent.
A new type of terrorism, which we term as
bio-cyber terrorism, could effectively take advantage of the
numerous possibilities offered by the IoBNT to control and interact
with the biological environment.
For example, Bio-NanoThings could be used to access the human body,
and either steal personal health-related information, or even create
new diseases.
Moreover, new types of viruses could be created
to hack into already deployed IoBNTs.
Research within the IoBNT field should necessarily address these
problems by combining the security assurance methods applied to
today's computer networks with security solutions developed through
evolution by nature, such as the human immune system.
The realization of localization and tracking
techniques within the IoBNT, in a similar way as realized in
wireless sensor networks (WSNs), could enable important
applications related, for example, to the monitoring of disease
locations in the body or identification of the location and
distribution of toxic agents in an environment.
One solution could come from the engineering of chemotaxis in Bio-NanoThings,
based on the aforementioned capability of bacteria to localize and
track sources of particular types of molecules, which could be, for
example, biomarkers released by cancerous or infected cells.
In line with the vision of "all-Things
connected," an ultimate goal is to interconnect the paradigms of
IoBNT and IoNT to the IoT.
A challenge of incorporating nanoscale devices is the large quantity
of information that will emerge, taking the challenges of managing
"Big- Data" to a new level.
Besides the increase in the quantity of data, new
services will need to be designed to semantically map between
different types of data that IoBNT and IoNT will feed to the IoT.
New service discovery solutions will also be
required to search deep into the biological environments and
interact with engineered biological entities to actuate or collect
information.
Conclusion
While the Internet of Things (IoT) is enabling the pervasive
connectivity of real-world physical elements among themselves and to
the Internet, the Internet of NanoThings proposes to push the limits
of this concept to nanotechnology-enabled nanoscale devices, which
can be easily concealed, implanted, and scattered in the
environment.
In this article we introduced the further concept
of Internet of Bio-NanoThings, where synthetic biology and
nanotechnology are combined to develop Things based on the control,
reuse, modification, and reengineering of biological cells.
This article outlined the challenges that will be faced to realize
these Things and, more importantly, to enable their communication
and networking, with paradigm-shifting techniques for the fields of
communication and network engineering.
We believe that the IoBNT research field, while
still in its infancy, will result in a game-changer technology for
the society of tomorrow.
References
-
I. F. Akyildiz and J. M. Jornet, "The
Internet of Nano-Things," IEEE Wireless Commun., vol. 17,
no. 6, Dec.2010, pp. 58–63.
-
L. J. Kahl and D. Endy, "A Survey of
Enabling Technologies in Synthetic Biology," J. Biological
Engineering, vol. 7, no. 1, May 2013, p. 13.
-
I. F. Akyildiz, F. Brunetti, and C.
Blazquez, "Nanonetworks: A New Communication Paradigm,"
Computer Networks, vol. 52, no. 12, Aug. 2008, pp. 2260–79.
-
D. L. Nelson and M. M. Cox, Lehninger
Principles of Biochemistry, W. H. Freeman, 2005, pp. 425–29.
-
C. J. Myers, Engineering Genetic
Circuits, Chapman & Hall/CRC, Mathematical and Computational
Biology Series, 2009.
-
D. Baker et al., Engineering Life:
Building A Fab for Biology, Scientific American, vol. 294,
no. 6, June 2006, pp. 44–51.
-
F. Wu and C. Tan, "The Engineering of
artificial Cellular Nanosystems Using Synthetic Biology
Approaches," WIREs Nanomedicine and Nanobiotech, vol. 6, no.
4, July/Aug. 2014.
-
M. Pierobon,"A Systems-Theoretic
Model of a Biological Circuit for Molecular Communication in
Nanonetworks," Nano Communication Networks (Elsevier), vol.
5, no. 1–2, Mar.–June 2014, pp. 25–34.
-
M. Gregori and I. F. Akyildiz, "A
New NanoNetwork Architecture using Flagellated Bacteria and
Catalytic Nanomotors," IEEE JSAC, vol. 28, no. 4, May 2010,
pp. 612–19.
-
M. Barros et al., "Transmission Protocols
for Calcium-Signaling-based Molecular Communications in
Deformable Cellular Tissue," IEEE Trans. Nanotechnology,
vol. 13, no. 4, May 2014, pp. 779–88.
-
M. J. Moore, T. Suda, and K. Oiwa,
"Molecular Communication: Modeling Noise Effects on
Information Rate," IEEE Trans. Nanobioscience, vol. 8, no.
2, June 2009, pp. 169–80.
-
Y. Chahibi et al., "A Molecular
Communication System Model for Particulate Drug Delivery
Systems," IEEE Trans. Biomedical Engineering, vol. 60, no.
12, 2013, pp. 3468–83.
-
M. Pierobon and I. F. Akyildiz,
"Fundamentals of Diffusion-Based Molecular Communication in
Nanonetworks," Now Publishers Inc, ISBN-10: 1601988168,
ISBN-13: 978- 1601988164, Apr. 2014, 164 pages.
-
I. F. Akyildiz et al., "MoNaCo:
Fundamentals of Molecular Nano-Communication Networks," IEEE
Wireless Commun. Mag., vol. 19, no. 5, Oct. 2012, pp. 12–18.
-
C. R. Yonzon et al., "Towards Advanced
Chemical and Biological Nanosensors – An Overview," Talanta,
vol. 67, no. 3, Sept. 2005, pp. 438–48.
Biographies
I. F. AKYILDIZ is Ken Byers Chair
Professor in Telecommunications with the School of Electrical
and Computer Engineering, Georgia Institute of Technology,
Atlanta, the Director of the Broadband Wireless Networking (BWN)
Laboratory and the Chair of the Telecommunication Group at
Georgia Tech. Since 2013 he has been a FiDiPro Professor
(Finland Distinguished Professor Program (FiDiPro) supported by
the Academy of Finland) in the Department of Electronics and
Communications Engineering at Tampere University of Technology,
Finland. He is an IEEE Fellow (1996) and an ACM Fellow (1997).
He has received numerous awards from IEEE and ACM. His current
research interests are in nanonetworks, TeraHertz band
communication networks, 5G cellular systems, and wireless sensor
networks.
M. PIEROBON received the Ph.D. degree in electrical and
computer engineering from the Georgia Institute of Technology,
Atlanta, GA, in 2013, and his M.S. degree in telecommunication
engineering from the Politecnico di Milano, Milan, Italy, in
2005. Currently he is an assistant professor with the Department
of Computer Science & Engineering at the University of Nebraska-
Lincoln. He is an editor of the IEEE Transactions on
Communications. He is a member of IEEE, ACM, and ACS. His
current research interests are in molecular communication theory
for nanonetworks, communication engineering applied to
intelligent drug delivery systems, and telecommunication
engineering applied to cell-to-cell communications.
S. BALASUBRAMANIAM received his bachelor (electrical and
electronic engineering) and Ph.D. degrees from the University of
Queensland in 1998 and 2005, respectively, and the master's
(computer and communication engineering) degree in 1999 from
Queensland University of Technology. He is currently a senior
research fellow at the Nano Communication Centre, Department of
Electronic and Communication Engineering, Tampere University of
Technology (TUT), Finland. He was the TPC co-chair for ACM
NANOCOM 2014 and IEEE MoNaCom 2011. He is currently an editor
for IEEE Internet of Things and Elsevier's Nano Communication
Networks. His current research interests include bio-inspired
communication networks and molecular communication.
Y. KOUCHERYAVY (evgeni.kucheryavy@tut.fi) is a full
professor and lab director in the Department of Electronics and
Communications Engineering at the Tampere University of
Technology (TUT), Finland. He received his Ph.D. degree (2004)
from the TUT. He is the author of numerous publications in the
field of advanced wired and wireless networking and
communications. His current research interests include various
aspects of heterogeneous wireless communication networks and
systems, the Internet of Things and its standardization, and
nano communications. He is an associate technical editor of IEEE
Communications Magazine and an editor of IEEE Communications
Surveys and Tutorials.
|







