|

by Max Jones
July 03, 2024
from
UnlimitedHangout Website
|
Max Jones
is the producer for The Chris Hedges Report, and a staff
writer and video producer for ScheerPost.
After graduating summa cum
laude from the University of Southern California in 2023, where he
studied communications and screenwriting, his work has been
published in ScheerPost and republished at Popular Resistance.
He
has also interviewed a wide range of figures such as John Kiriakou,
Ray McGovern and David Hundeyin.
He continues to write fictional
stories for the big screen, has directed an independent short film
and produced multiple viral videos for both The Chris Hedges Report
and ScheerPost. |

In the face of a potentially
industry-ending slew of patent cliffs,
Big Pharma has begun
acquiring biotechnology companies
to stave off
collapse.
To get these drugs to market,
the industry is pursuing the
only solution left
for their dying model:
a full takeover of the WHO
to capture the global regulatory system.
Big Pharma must soon confront an industry-wide hazard that reaches
magnitudes far greater than the typical concerns of corporate profit
margins and business politics.
Through years of industry
consolidation, it has essentially made itself "too big to fail."
Only now, the model which it once could never fail within - that is,
the practice of obtaining patent exclusivity over drugs that are
approved through clinical trials and regulations - has become
obsolete, even impossible, under the current conditions of the
industry.
In this new climate, the trials and regulations Big Pharma once
successfully navigated may very well lead to its total demise.
However, the pharmaceutical sector has set its eyes on the only
solution that can keep its money and power intact; the full takeover
of the public sector, specifically the World Health Organization
(WHO), and the regulatory system that now holds the entire market
hostage.
The problem begins with the looming financial
threat that the top 20 Big Pharma companies face: from now until
2030,
$180 billion in sales will be at risk. This threat, called a
patent cliff, is a regularly occurring problem for the
pharmaceutical industry.
Big Pharma has long made its money by
attaining patent exclusivity of certain drugs, thereby monopolizing
all potential profits made off of them for a finite amount of time.
When that patent exclusivity ends, the drug rolls head first off of
a "patent cliff," and tens of billions of dollars in revenue are put
at risk.
Typically, companies
address patent cliffs through mergers and acquisitions (M&A)of
other, often smaller drug companies that produce products with
market potential.
This time around,
however, according to Biopharma Dive,
"after years of
industry consolidation, there are not many major large drugmakers
left as attractive merger targets."
In other words, Big Pharma has
become "too big to fail" and - over the next 6 years - faces a new
round of potentially disastrous patent cliffs.
Further, traditional
chemical drugs already exist for many diseases and regulators have
increased approval standards for them, delaying the time in which
new products obtained from M&As can be taken to market.
As a result, companies facing patent cliffs have
shifted their patent cliff response efforts to acquiring biotech and
biologic companies that produce products that, compared to their
more typical chemical-based counterparts, are more
complex,
unpredictable and difficult and expensive to make.
The race for
future blockbuster drugs will therefore
take place "in big drugmakers' own laboratories or in those of
smaller biotechnology companies" as opposed to mergers with other
large corporations.
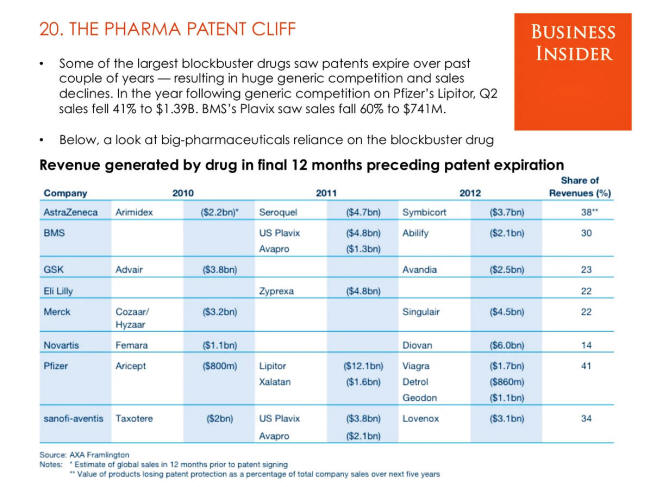
The Pharma Patent Cliff
SOURCE: Business Insider
To understand what makes biologics so complex and
unpredictable, their vast difference in function and origin compared
to chemical-based drugs must be understood.
Biologics are
taken from different natural sources, such as humans, animals or
microorganisms and,
"may be produced by biotechnology... and other
cutting-edge technologies."
While chemical drugs activate one's
entire immune system in a general manner, biologics
target,
"certain proteins or cells in your immune system to
create specific responses",
...hence the use of cutting-edge technology
to achieve these more specific medical goals.
There are multiple reasons Pharma companies might
be interested in biotech - but three are clear from a market
perspective.
The complex nature of biologics makes them impossible
to replicate in the way a typical chemical-based drug would be,
forcing companies to make "biosimilar" versions of the drugs as
opposed to generics.
This means that biosimilar versions of
biologics
cannot be interchanged without consequence during a patient's
treatment in the way a generic could be, for example.
Their costly
development also makes their off-label counterparts more difficult
to sell at significant discounts, making biosimilars not as
financially appealing to consumers as generic drugs.
There are also
complex regulatory obstacles in getting biosimilars to market,
even after they're approved by the Food and Drug Administration
(FDA).
These attractive characteristics have made biotech the
hopeful solution of the coming patent cliff Big Pharma companies are
preparing to face, with a slew of top Pharma corporations acquiring
gene editing, antibody-drug conjugate and other biotech companies to
offset their potential losses (see
here,
here,
here,
here,
here and
here).
On face value, this investment in biologics
appears like a typical patent cliff response; purchasing new
companies that produce drugs with "blockbuster" potential, and
hoping they will alleviate the incoming losses.
However, there are
significant obstacles biotech/biologics face from a market
perspective that make Pharma's investment in them a significant
shift in the industry - the unpredictability of biologics has proven
to make them consistently unsafe.
The
mRNA COVID-19 vaccines, which were biologic
drugs, were associated with an excess
risk of serious adverse effects, and can cause fatal
myocarditis.
CRISPR, the most popular gene editing
biotechnology, often silences and activates genes it isn't meant to,
leading to adverse effects such as cancer (also
see here).
Antibody-drug
conjugates induce serious adverse events 46.1% of the time according
to a
study by Zhu et al. and are
significantly associated with sepsis in cancer patients, which
increases mortality.
Thus, these unattractive characteristics make it
more difficult for biologics/biotech to be successful within the
conventional regulatory framework under which most medicine
development currently operates.
Yet, persuading consumers that an
unpredictable, highly technical drug is safe and effective may also
prove difficult.
Luckily for Big Pharma, the World Health
Organization and its massively endowed public-private partners are
pursuing an unprecedented legal process that would cement loopholes
that could solve these significant market challenges of at least
some biotechnologies, and which already proved to make Big Pharma
record profits during the COVID-19 'pandemic', when normal regulatory
hurdles were removed.
The mRNA COVID-19 vaccines quickly became Big
Pharma's highest selling annual
market success ever.
As a result of the COVID-19 vaccines,
Pfizer made $35 billion, while competitors BioNTech and Moderna
raked in $20 billion each
in 2021 and 2022.
Bill Gates turned his $55 million investment
in BioNTech
into $550 million. 70% of the US population is now
fully vaccinated, as well as 70% of the
world population.
This could not have been achieved without the
fast-tracked, deregulated development and mandated consumption of
the experimental drugs - a plan that was, regarding fast-tracked
development (not mandates), outlined in the
Pentagon–run
Operation Warp Speed and legally authorized by the FDA's
emergency use authorization and the
WHO's Emergency Use Listing.
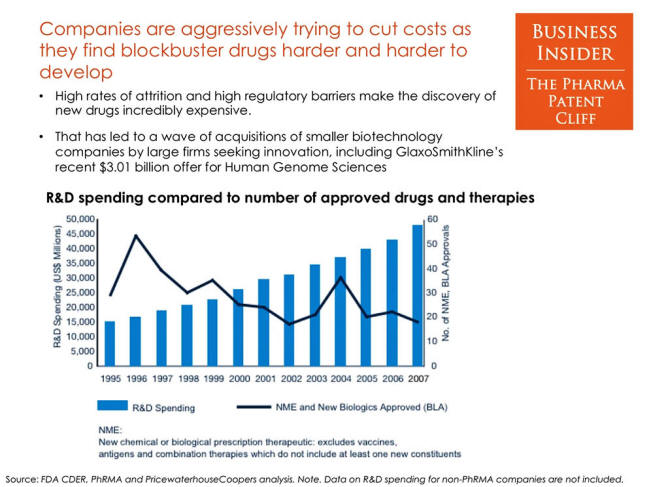
Companies aggressively
trying to cut costs
SOURCE: Business Insider
These "emergency use" labels allowed Pharma
companies to override the standards regularly associated with the
tenuous process of getting a drug passed through lengthy clinical
trials, which previously almost caused biotech company Moderna
to collapse before the 'pandemic'.
A story that perfectly
demonstrates the urgent need for biotech companies to eliminate the
regulatory standards typically required for medical products, and
the precedent the public sector established in providing a path for
this regulatory obfuscation.
Founded roughly ten years before the 'pandemic', Moderna, which was highly valued for many years based on its promise
to produce products for rare diseases that required multiple doses
throughout a patient's life, had failed to deliver any products at
all by early 2020. In addition, it had not even been able to prove
in clinical trials that it could produce safe and effective mRNA
products.
Their allegedly revolutionary drug technologies
were consistently plagued by toxicity issues when dosed to
"effective" amounts, and were ineffective when dosed to "safe"
amounts.
The safety problems were so bad that Moderna was forced to
abandon its key mRNA-based biologic treatment that it had used to
raise most of its capital and which justified the company's high
valuation after
whistleblowers shed light on the fact that it couldn't even make
it to human trials.
These safety issues, among other damning
political ones, set up Moderna for an imminent collapse right up to
before the 'pandemic', with funding drying up and the company being
instructed to "stretch every dollar" and reduce expenses, as well as
a declining stock price that was paired with key executives leaving
the company at critical junctures in the lead-up to 2020.
But when news of a virus out of Wuhan emerged in
late 2019, while many worried of a coming 'pandemic', Moderna's CEO,
Stéphane Bancel, had a golden opportunity placed in his failing
company's lap.
At the time, the deputy director of the Vaccine
Research Center at the National Institutes of Health, Barney Graham,
was already preparing the NIH to develop vaccine candidates for
the coming virus.
Much to Moderna's relief, the company had already
been working on "bring[ing] a whole new class of vaccines to market'
with Graham in the years prior to the 'pandemic'.
This
relationship, among other events described in Unlimited Hangout's
exposé on Moderna, eventually led Graham
to propose to the Moderna CEO the opportunity to use the coming
'pandemic' virus,
"to test the company's accelerated vaccine-making
capabilities," before any experts had officially declared that a
vaccine was the solution to the 'pandemic'.
This agreement, which would manifest in the
coming months, put Moderna on the frontlines of the US government's
accelerated vaccine program, "Operation Warp Speed," and rewarded
Moderna with its first and only product; the COVID-19 mRNA vaccines,
which
generated billions in profit.
Notably, the previously failing
and stagnant company was only able to launch this product due to the
"emergency" removal of the same regulatory hurdles that had
previously prevented Moderna from taking any of its drug candidates
to market.
Thus, the COVID-19 vaccines went to market in
just
326 days, a fraction of the
10-15 years it typically takes vaccines to go to market.
This
timing was critical to the Operation Warp Speed goal of vaccinating
the entire American population - releasing the vaccine in the heat
of the 'pandemic', before lockdowns and social restrictions had ended,
likely made people more concerned with ending the 'pandemic' than the
regulatory criterion of the drug.
As a result, whether
state-mandated in countries like Austria or
job-dependent in the U.S., many people accepted vaccine mandates
without question for a drug that was rushed to market.
The rapid development and mandated consumption of
experimental drugs, a strategy which was
first adopted by the military to respond to bioweapons attacks,
has now been internationally legitimized by the WHO, as it recently
approved
critical revisions to the
International Health Regulations and continues to draft its
recently shelved
WHO CA+ treaty.
While the WHO has
claimed that these conventions are being drafted to prepare the
global population for a future with an ever-increasing incidence of
deadly 'pandemic's (the next of which they refer to as
"Disease X"),
the core policies of these documents - driven by
the ideology of the Global Health Security doctrine and
the "One Health" agenda - would further codify
surveillance-heavy and emergency deregulatory measures that would
create a massively profitable and permanent market for certain
products in Big Pharma's new biotech arsenal.
Like it was during Operation Warp Speed, the US
remains on the frontline of the effort to accelerate the process of
getting biologic drugs to market under the guise of 'pandemic'
preparedness.
Just this week (July 2024), the United States
Department of Health and Human Services's (HHS) Biomedical Advanced
Research and Development Authority (BARDA), which
aims to,
"respond to 21st century health security threats,"
granted
$176 million to Moderna to
"accelerate development
of a 'pandemic' influenza vaccine that could be used
to treat bird flu in people, as concern grows about cases in
dairy cows across the country".
This is likely the first government contract
awarded to a biotech company since the COVID-19 'pandemic' with the
specific intention of "accelerating" vaccine development against an
allegedly impending 'pandemic' virus (other
biotech companies
have been awarded contracts for 'pandemic' preparedness, however).
The tactic of preemptive development of
'pandemic' drugs, and their
accelerated path to market, is a key component of the new WHO
conventions and an essential factor in the viability of the biotech
'pandemic' market - and may now provide Moderna with its second
product ever.
Members of the US military
unbox vials of
Moderna's COVID-19 vaccine in 2021
SOURCE: US Army
The biotech 'pandemic' market, as described above,
will not be one that depends on the free will of consumers to opt in
and out of products - but instead relies on tactics of forced
consumption and manipulation of regulatory paradigms.
At the
forefront of this push are the WHO's public-private-partners /
private stakeholders, who directly shape and benefit from this
policy.
Their influence has, in effect, turned the WHO into an arm
of Big Pharma, one so powerful that it already demonstrated its
ability to morph the entire international regulatory process for the
benefit of the pharmaceutical industry during the COVID-19
'pandemic'.
These new laws will solidify that influence further, and legally
bond the entire global community to the permanent 'pandemic' market
being built on Big Pharma's behalf.
Who funds the WHO?
The WHO is
funded
through regular contributions from member states - which make up
20% of its funding - and private stakeholders - which make up the
other 80% and thus the bulk of its finances.
The organization's
overwhelming reliance on private funding has made it vulnerable to
vast influence from its stakeholders, providing an avenue for
private actors to dictate WHO policy, sit on crucial committees, run
entire distribution programs and even occupy top bureaucratic
positions.
The private sector influence on the WHO
materialized most tangibly and consequentially during the COVID-19
'pandemic', when the
pre-'pandemic' goals of WHO public-private partners like,
-
the Gates-funded
Coalition for Epidemic Preparedness Innovations (CEPI) to "speed the
development of vaccines"
-
Bill Gates (who advocated
developing vaccines in 90 days or less) heavily influenced
global 'pandemic' response for WHO member states...
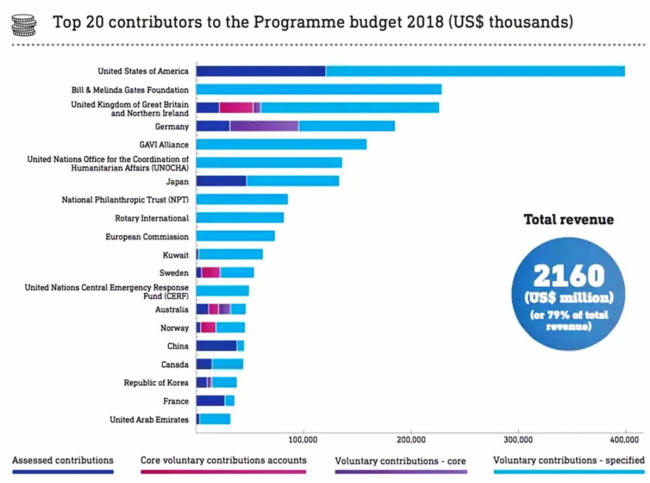
WHO Funding Sources from
2018
SOURCE: World Economic Forum
Similarly, public sector bureaucrats such as Dr.
Anthony Fauci, Obama's former FDA commissioner
Margaret Hamburg, and
Rick Bright of BARDA and the
Rockefeller Foundation, all advocated in an
Oct. 2019 panel for,
the creation of a new system that emphasized
"speed and effectiveness" and "fast" vaccines...
Fauci declared the
importance of changing people's perception of influenza as a mild
disease and doing so in "a disruptive [and iterative] way" and, as
Whitney Webb noted in her
exposé on Moderna,
"[Bright] said the best way to 'disrupt' the
vaccine field in favor of 'faster' vaccines would be the emergence
of,
'an entity of excitement out there that's completely disruptive,
that's not beholden to bureaucratic strings and processes.'
He
later very directly said that by 'faster' vaccines he meant mRNA
vaccines."
Notably, whether or not member states had in
place US/UK-like emergency use authorization laws, which allow for
the fast-tracked development and distribution of experimental,
unapproved drugs in the face of emergencies like pandemics, member
countries universally adopted them to develop and/or distribute the
COVID-19 vaccines - a quite "disruptive" and "fast" way of getting
these vaccines to market, done exactly by not making their
development,
"not beholden to bureaucratic strings and processes."
Before this emergency deregulation was adopted,
the WHO had to grant this unprecedented effort, and the unapproved
vaccines, legitimacy through its
Emergency Use Listing procedure (EUL), which it uses to "assess
and list" unapproved drugs,
"with the ultimate aim of expediting the
availability of these products to people affected by a public health
emergency."
While the EUL is officially an advisory label
meant to help member states make their own decisions, in reality it
has de facto legal consequences that significantly
influence the global economy - mainly through the role EUL plays in
the WHO's global vaccine distribution program, COVAX.
According to
the WHO's website, COVAX's, "EUL / prequalification programs
ensured harmonized review and authorization across member states,"
highlighting the legal and regulatory influence of the WHO's EUL.
While COVAX operates as a crucial delivery system
for WHO-approved medical products during 'pandemic's, it also serves
as perhaps the clearest example of the scale and inner-workings of
financial corruption in the WHO. COVAX, like many WHO operations, is
a public-private-partnership, or a long-term collaboration between
the WHO and private companies.
Bill Gates - who described his $10 billion
investment in vaccines which yielded a $200 billion return as his "best
investment" - is deeply financially entangled in the WHO's
COVAX.
COVAX's
stated goal during the COVID-19 'pandemic' was to,
"accelerate the
development, production, and equitable access to COVID-19 tests,
treatments, and vaccines."
It was co-led by the Bill Gates-founded Gavi, the WHO, CEPI and the Gates-funded
UNICEF.
Gavi,
CEPI, the
Gates Foundation and
UNICEF have all pushed for accelerated vaccine development
before the 'pandemic'. Notably, Gavi's stated goal is to create "healthy
markets" for vaccines
by,
"encourag[ing] manufacturers to lower vaccine prices for the
poorest countries in return for long-term, high-volume and
predictable demand for those countries".
COVAX also
developed a "No-Fault
Compensation Program" that worked to,
"[reduce] the risk of
litigation for [vaccine] manufacturers" by "indemnify[ing]
manufacturers against any financial losses they may incur from the
deployment and use of these vaccines."
In other words,
the WHO
worked to exonerate Big Pharma companies of legal and financial
liability from adverse events produced by its rapidly approved COVID
vaccines.
Thus, Big Pharma, with the WHO's assistance, was not only
able to rush troubled products to market, but with complete impunity
for any harm those products may cause.
Further, the founder of CEPI and former director
of the Wellcome Trust (both of which are large funders of the WHO),
Jeremy Farrar, was made Chief Scientist of the WHO in December
2022 - further
entrenching the goals of Gates and CEPI into the WHO's policy
agenda.
Even thirteen of the fifteen members on the WHO's
Strategic Advisory Group of Experts on Immunization (SAGE) either
hail directly from careers at companies that are private
stakeholders of the WHO, most often the Wellcome Trust or NGOs and
institutions funded by Bill Gates or the Gates Foundation.
The ongoing amendments to the IHR and drafting of
the WHO CA+ treaty reflect the latest effort of the WHO's
public-private-partners to solidify their global influence by using
the United Nations (UN) organization as a proxy, codifying their
policy agenda under the auspices of the most recognized
international health organization in the world.
While the
conventions purport to further the alleged international interest of
'pandemic' preparedness, the measures they call for - which already
proved to make Big Pharma record profits during the COVID-19
'pandemic' despite no real public health benefit - would enshrine the
disasters of COVID-era vaccine policy (rushed, under-tested Pharma
products imposed on the public through mandates) as the default
response to public health concerns, whether deemed more dangerous or
minor in comparison to COVID-19.
Solidifying COVID-19 Measures,
Paving the Way for the 'pandemic' Market
The central policy measures of the IHR amendments
and the WHO CA+ treaty would create a permanent financial market
centered around 'pandemic' preparedness and response.
The core
ideology that runs through both of these conventions is the "One
Health" agenda.
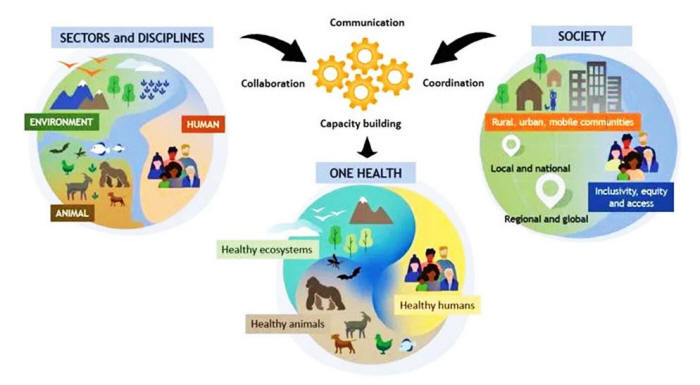
The "One Health"
Approach
SOURCE: The One Health Commission
According to the WHO's
website, a "One Health" approach to
'pandemic' preparedness and
response plans to,
"[link] humans, animals and the environment" in
order to "address the full spectrum of disease control - from
prevention to detection, preparedness, response and management - and
contribute to global health security."
In other words,
it requires
full-scale surveillance of the human-animal environment, both before
'pandemic's for purposes of prevention and preparedness, and during
'pandemic's for the purpose of response.
Also vital to the One Health
model is the interoperability and accessibility of data (gathered
through surveillance) - or as the WHO puts it,
"shared and effective
governance, communication, collaboration and coordination."
From a business perspective, the One Health
agenda would create a cyclical market built on two dominant
principles: constant surveillance of pathogens with "'pandemic'
potential," and R&D on medical countermeasures to these pathogens.
This R&D then comes to market through the implementation of
regulatory policies for the development and distribution of
unapproved, experimental medical products.
The recently approved IHR
amendments have already cemented these principles into international
law. The continued drafting of the WHO CA+ seeks to as well.
I.
Biosurveillance on Pathogens w/
'pandemic' Potential
Both conventions specifically call for member
states to be legally required to build infrastructure to conduct
biosurveillance on entire populations.
For example, the WHO CA+
requires member states to,
"commit to promote a One Health approach
for 'pandemic' prevention, preparedness and response that
is... integrated, coordinated and collaborative among relevant actors
and sectors," and to strengthen 'pandemic' prevention through
"collaborative surveillance" and "vector-borne disease surveillance
and prevention",
...among other similar provisions.
Similarly, a new addition to Annex 1 of the IHR
calls on states to,
"develop, strengthen and maintain the core
capacities to coordinate with and support the local level in
preparing for and responding to public health risks... including in
relation to: surveillance... implementation of
control measures... [and] addressing misinformation and
disinformation; and logistics."
This arguably reiterates a provision
already established in the previously approved 2005 IHR, which says
that member states must,
"develop, strengthen and maintain... the
capacity to detect, assess, notify and report events in accordance
with these Regulations."
Notably, however, the new IHR links
surveillance to several other "core capacities" - including,
"implementation of control measures" and "addressing [mis/disinformation]."
While the approved IHR does not directly mention
surveillance at the human-animal interface, the WHO remains
committed to its
One Health Initiative.
Both of these statutes together would
obligate member states to utilize biosurveillance tools to monitor
dangerous pathogens.
The latest WHO CA+ treaty draft goes further on
this front than the IHR, with one of its goals being to reaffirm,
"the importance of multisectoral collaboration at national, regional
and international levels to safeguard human health, including
through a One Health approach."
Under the draft, member states would
also be required to,
"promote a One Health approach" for
'pandemic'
preparedness and response through "coherent, integrated, coordinated
and collaborative among all relevant organizations, sectors and
actors, taking into account national circumstances."
Further, they
must identify, presumably through building up surveillance
capacities,
"the drivers of 'pandemic's and the emergence or
reemergence of disease at the human-animal-environment interface... "
It turns out that the WHO's private stakeholders
began funding initiatives towards this end years before the COVID-19
'pandemic'.
The Wellcome Trust, whose former director Jeremy Farrar is
now Chief Scientist at the WHO, and the Gates Foundation
funded an AMR Industry Declaration effort in 2016 which included
an,
"industry commitment to share antimicrobial resistance
surveillance data."
As part of this commitment, pharmaceutical
companies
agreed to,
"continue to share the surveillance data we generate
with public health bodies and healthcare professionals... inform
appropriate antibiotic and vaccine use and, over time, thereby help
increase surveillance capabilities globally."

Jeremy Farrar
attends
the World
Economic Forum
annual meeting in
2023
SOURCE: Health Policy Watch
The Wellcome Trust stated, referring to this
initiative, that,
"There is a clear need for the public and private
sectors to share the data they gather from local and global
antibiotic surveillance studies."
In a 2016
press release, the Wellcome Trust issued a press release
advocating for,
"research data gathered during... future public health
emergencies, to be made available as rapidly and openly as
possible."
Similarly, in a 2017 statement Bill Gates
stressed the importance of biosurveillance data sharing:
"We
also have to ensure that every country is conducting routine
surveillance to gather and verify disease outbreak intelligence... we
must ensure that countries share information in a timely way, and
that there are adequate laboratory resources to identify and monitor
suspect pathogens."
This kind of biosurveillance is not something
novel or technologically distant.
The Palantir and Department of
Health and Human Services (HHS) program "Protect"
surveilled wastewater treatment plants across the United States,
"to
predict new COVID-19 cases five to eleven days before an outbreak"
as part of a mass data collection plan during the 'pandemic'.
The
program was actually a resurrection of
a post-9/11 surveillance program that had been housed at the
Pentagon's DARPA, but was quickly scrapped due to privacy concerns.
Under Protect, the Trump administration forced US hospitals to enter
"all data on Covid-19 cases and patient information directly into HHS Protect" by
threatening Medicare and Medicaid funding for any hospitals who
did not comply.
Palantir, which obtained all of HHS' COVID data, was
created with
significant CIA involvement in order to obtain predictive
intelligence from mass civilian surveillance to stop threats - from
terror attacks to public health emergencies - before they happen.
Predicting outbreaks through biosurveillance is
crucial to fueling the research and development (R&D) phase of
'pandemic' preparedness and response - and a critical opportunity for
biotech products to be developed and administered outside the
typical regulatory system.
It also allows them to be developed and
marketed preemptively, meaning that - in the wrong hands - products
could be sped through development and forced on the public without
any material public health threat at all.
Merely the possibility of
a threat could potentially trigger the same style of response
observed during COVID-19 or, worse still, the intentional release of
the very pathogens targeted by "preemptive" drug/vaccine development
could be incentivized.
II.
Research and Development
While the recently passed IHR amendments do not
mandate that state parties collaborate on "research and development
cooperation [and] technological and information sharing," as
previous drafts
did, the WHO Director-General is now officially required to,
"support States Parties, upon their
request,... to
promote research and development and strengthen local production of
quality, safe and effective relevant health products, and facilitate
other measures relevant for the full implementation of this
provision".
In the
latest draft of the WHO CA+, member states are required to,
"cooperate to build, strengthen and
sustain geographically diverse capacities and institutions
for research and development... based on a
shared agenda" and "promote research collaboration and access to
research through open science approaches for the rapid sharing of
information and results, especially during 'pandemic's."
This would expand the WHO's 2014
CEPI-partnered program, Research and Development Blueprint for
Emerging Pathogens (R&D Blueprint) that aims to,
"reduce the time
between the declaration of a ['pandemic'] and the availability of
effective tests, vaccines and medicines."
However, the Blueprint does this not only through
R&D as a means of 'pandemic' response, but also
'pandemic' prevention through conducting R&D on diseases that,
"are
likely to cause epidemics in the future."
The R&D Blueprint is
therefore an extension of the WHO's biosurveillance measures, as it
will commission research and development of medical products for
pathogens that are detected through "One Health" style
surveillance.
This kind of preventive R&D has caused experts on
the WHO CA+ and IHR to
raise serious concerns about its potential to incentivize
gain-of-function (GoF) research.
Since GoF research is
considered dual-use, which means it can be used for both preventive
'pandemic' measures and as offensive biological weapons, it is
possible the allegedly defensive/peaceful R&D the WHO is advocating
for could also be used to develop biological weapons.
The WHO is clearly aware of this risk. In a WHO
BioHub
safety document, for example, it recommends that labs affiliated
with the WHO's data-sharing pathogen program develop a biosecurity
plan that,
"include[s] the measures to be implemented to prevent the
theft, misuse and intentional release of hazardous biological
agents."
The latest draft of the WHO CA+ also states that member
states must implement,
"laboratory biological risk management in
order to prevent the accidental exposure to, the misuse of or the
inadvertent release of pathogens."
Whether or not these leaks are accidental or
intentional, the
highly-likely origin of COVID-19 being a lab leak speaks to the
ability for these pathogens to cause severe global disruption.
This
raises concerns about whether or not conducting more preventive R&D
on dangerous pathogens is actually being done for the purpose of
"preparedness," especially considering that preemptive R&D
will presumably play a crucial role in getting Big
Pharma-owned 'pandemic' drugs to market.
Further, considering CEPI founder and former
Wellcome Trust director Jeremy Farrar's position as Chief Scientist
of the WHO, and the
leading role that CEPI played in funding R&D for the COVID-19
vaccines as well as the role they
continue to play in funding vaccines for future coronaviruses
and against the allegedly forthcoming "Disease X",
plus their
unique funding model
which pools from "several investors," which
have included the
Gates Foundation, the Wellcome Trust and
The World Bank,
...it is highly likely that the R&D on these future
pathogens with 'pandemic' potential will be carried out by CEPI and
other WHO public-private partnerships with financial conflicts of
interest.
The Wellcome Trust and the Gates Foundation also
provided significant funds to
start CEPI, which launched in 2017 with the goal of,
"shorten[ing] the time it takes to develop new vaccines to protect
against viruses that emerge suddenly as public health threats... "
Key
to shortening the development time of vaccines is the process of
deregulation, which permits the rapid development and distribution
of unapproved medical products - also a vital component of the new IHR
and the WHO CA+.
III.
Regulatory Pathways
The new IHR provides sufficient regulatory
opportunities for unapproved medical products to make their way to
market in the face of a 'pandemic' - a perfect deus ex machina
for the risky biotech products that make up Big Pharma's new
acquisitions.
Under the new international convention, the
Director-General is required to provide, when requested by member
states, documents,
"related to a specific relevant health product"
which would be "provided to WHO by the [drug] manufacturer... for the
purpose of facilitating
regulatory
evaluation and authorization by the State Party".
While the exact implications of this provision
are unclear, international law professor Dr. Amrei Müller,
co-founder of the Global Health Responsibility Agency and expert on
the WHO CA+ and IHR, told Unlimited Hangout that she
suspects this,
"provision once more aims at speeding up emergency
authorizations at the domestic level during a ['pandemic']
of WHO-recommended 'relevant health products' that are
investigational," noting that the WHO does not specify whether or
not these "relevant health products" must be fully licensed.
Similarly, the WHO Director-General is now
required to,
"support State Parties, upon request, in scaling up and
geographically diversifying the production of relevant health
products... "
The documents do not prohibit the WHO from influencing
domestic regulatory laws in order to "[scale] up" the production of
health products.
The WHO CA+ more forcefully seeks to influence
domestic regulation policy to this end. It calls on member states to,
"take steps to ensure that [they have] the
legal,
administrative and financial frameworks in place to support
emergency regulatory authorizations for the effective and
timely approval of 'pandemic'-related health products during a
'pandemic'",
...including through "technical assistance
and cooperation with WHO" - an attempt to mandate that member states
implement emergency use authorization policies at the domestic level
to carry out the rapid response agenda of the WHO's
public-private-partners.
These provisions expand on the goals of the WHO's
public-private program, the
Access to COVID-19 Tools (ACT) Accelerator,
"to accelerate
development, production, and equitable access to COVID-19 tests,
treatments, and vaccines" to diseases beyond COVID-19.
The
ACT Accelerator, of which COVAX is part, acted as a partnership
that included,
-
the WHO
-
Gates Foundation
-
GAVI
-
Wellcome Trust
-
the World Bank,
...all substantial funders of the WHO and partners of
the Gates foundation (including the
World Bank and
Wellcome Trust).
Notably, the World Bank and the Gates
Foundation funded a 2017 World Organization for Animal Health
program to develop "vaccine banks" which
sought to,
"[enable] the rapid supply of emergency stocks of
vaccines... in order to vaccinate targeted animal populations at
risk."

COVAX COVID-19
vaccines
are unloaded in
2021
SOURCE: CNN Español
A 2018 World Bank
policy document, which the Gates Foundation contributed to,
describes its "'pandemic' Emergency Fund" which also involved a "CEPI
trust fund" in which The World Bank funded CEPI to develop vaccines
in order to,
"[accelerate] vaccine development against pathogens with
'pandemic' potential... "
Part of this "acceleration" involved helping,
"improve
regulatory capacity in low-income countries and prepare
countries and sites to conduct clinical trials... " in order to ensure
that low-income countries "rapidly and effectively
have access to life-saving vaccines".
The act of
"improving regulatory capacity" likely refers to altering the
regulatory laws of countries that don't have in place emergency
deregulatory programs, such as the US's emergency use authorization
or the FDA's
Investigational New Drugs Application, which allows drug
companies to apply for FDA approval to administer unapproved biotech
products to humans.
Yet, the funders of the WHO are also seeking
other, more "innovative," methods to push for accelerated drug
development.
When Jeremy Farrar was still the director of the Wellcome Trust, he launched an initiative led by two former Defense
Advanced Research Projects Agency (DARPA) directors called Wellcome
Leap.
Wellcome Leap, a de facto global iteration of DARPA,
aims to employ synthetically created human organs for testing
the effects of pharmaceutical products.
If successful, this testing
method could replace animal - or perhaps even human - drug trials
using "gene-edited or farmed organs," further reducing the time
it would take to bring drugs to market.
It seems even the WHO
Review Committee tasked with critiquing the amendments, however,
believes the regulatory ambitions of the WHO may go too far
considering the organization's powers.
It noted that it,
"may be
inadvisable from a legal perspective to require that the WHO
develops such [domestic] regulatory guidelines [for member states]."
Obviously, these recommendations did not dissuade the WHO from
implementing deregulation opportunities for member states in the new IHR, nor from the WHO CA+.
The WHO's EUL of the COVID-19 vaccine, which
caused severe side effects, demonstrated the consequences of rushing
a drug to market under the guise of an emergency.
It also, however,
proved that providing a regulatory option for unapproved biologic
drugs that allowed developers to skip normal testing procedures
could be massively profitable.
In the midst of chaos and fear, the
standards of most people could be brushed aside in the name of
safety - a phenomenon perhaps most effectively demonstrated through
vaccine mandates.
IV.
Mandating Unapproved Medical
Products
In July 2023, the World Health Organization (WHO)
adopted the EU digital COVID-19 passport system,
"to establish a
global system that [would] help protect citizens across the world
from on-going and future health threats, including 'pandemic's."
Also known as an "immunity pass," the EU digital
COVID-19 vaccine passport dictated one's ability to travel based on
three criteria:
their vaccination record, negative test results
and records of previous infections.
While digitized vaccine
passports were not required, all contained,
"'a digitally signed QR
code' which [would] be scanned on entry to a country."
Before the
WHO adopted the system, 51 countries utilized the EU system to
dictate who could enter their borders - and presumably influence
many people to be vaccinated - from July 2021 to June 2023.
While a digital vaccine passport does not
function as a hard mandate in which every citizen of a given
population is forced to take a vaccine, it acts as a conditional
mandate - one which offers the illusion of choice, but - in reality
- restricts the civil liberties of those who do not comply.
For example, many countries such as Austria
implemented "soft" mandate tactics such as barring the
unvaccinated from eating at restaurants, cafes and going to
hairdressers.
The US enforced similar tactics, leaving it mostly up
to the private sector and
certain government bodies to enforce vaccine mandates, which
caused thousands of Americans to
lose their jobs for refusing to get the vaccine (also see
here).
The Biden administration also made
staff vaccination for Medicare and Medicaid hospitals mandatory
by threatening their federal funding, which caused
at least hundreds of healthcare workers to be fired or suspended
from their jobs.
Requiring proof of vaccination as a prerequisite
for otherwise standard rights - such as having a job, eating at a
restaurant or traveling - coerces people that otherwise would
not take the drug into doing so, and punishes those who express
their free will.
As Dr. Fauci said in a
book interview,
"it's been proven that when you make it
difficult for people in their lives, they lose their ideological
bullshit, and they get vaccinated."
The coercion permitted by the travel-based
mandate was actually already established in the 2005 IHR, which
allows member states to require,
"proof of vaccination or other
prophylaxis" for entry into a country "when necessary to determine
whether a public health risk exists; as a condition of entry for any
travelers seeking temporary or permanent residence," or to "achieve
the same or greater level of health protection than WHO
recommendations",
...essentially permitting member states to do
anything in the name of reducing the spread of disease.
The
new IHR expands on this by articulating the details of the
technology that will presumably be used to check medical records
during future 'pandemic's.
Specifically, it states that "health
documents" can be,
"issued in non-digital or digital format,
subject to the obligations of any State Party".
The IHR now also requires the WHO to,
"develop and update
[with member states]... technical guidance, including specifications or
standards related to the issuance and ascertainment of authenticity
of health documents, both in digital format and non-digital format."
The WHO's Chief Scientist, Jeremy Farrar, will
presumably have major influence on drafting these "standards"
related to vaccine passports.
In addition, his company CEPI is a
central
researcher and developer of vaccine technology against "Disease
X," increasing the likelihood that WHO private stakeholders will
influence the decision making around this process.

The WHO GDHCN
SOURCE: WHO on Twitter/X
The WHO Global Digital Health Certification
Network (GDHCN), which is currently being developed, is a good
indication of how these health checks will take place.
The GDHCN
expands the "regional networks" that the EU Digital COVID-19
Certificate system used, presumably on a "global" scale.
The GDHCN
aims to digitize vaccination certificates "within and across
borders" and act as a digital information hub for the storing of
traveling citizens' relevant medical records.
In other words,
it
will track "digitally signed health credentials (e.g. Immunization
cards, health records)," or which Big Pharma-developed
medicines/vaccines that citizens of the world have taken, in order
for member states to use that data to dictate the human rights of
travelers.
The WHO boasts
on its website of the system's "interoperability" - meaning its
capacity to work with other "existing regional networks" that have
already been established to verify health credentials.
Interoperability, perhaps the most significant attribute of the GDHCN, is a necessary component of the "One Health" approach to
'pandemic' preparedness.
It makes possible the WHO CA+ treaty goal of,
"multisectoral
collaboration at national, regional and international
levels to safeguard human health... ",
...and the mandate
for member states to,
"develop, strengthen [and]
implement... comprehensive multisectoral national 'pandemic' prevention"
through "collaborative surveillance," by providing a digital infrastructure for mass data sharing.
With this level of collaboration, governments can access all
citizens' relevant health records at the press of a button and
potentially share it with their private sector partners.
While interoperability is often
sold as a way to make a global system of surveillance and
identification "decentralized" through outsourcing these tasks to
multiple organizations, interoperability also allows data from
different governments and vendors to all be centralized and
accessible in the same global database.
In other words,
interoperability allows for de facto
centralization despite
many separate vendors, providing merely an illusion of
decentralization.
Conveniently, the GDHCN is being developed at the
same time that the UN is seeking to impose digital identification as
a "human right," or rather as a condition for accessing other human
rights, for the entire global citizenry by 2030, as established in
its Sustainable Development Goal 16.9.
The UN's digital ID goals are
being carried out through global public-private partnerships, mainly
the ID 2020 Alliance (now part of the Digital Impact Alliance).
The
ID2020 Alliance Manifesto states that,
"Individuals need a
trusted, verifiable way to prove who they are, both in the physical
world and online."
One of the ways it seeks to do this is by,
"providing a route to technical interoperability."
This "alliance" is also interestingly backed by
Bill Gates's Gavi - which raises a question of how much digital
identification, and thus digital health passports, are part of the
promotion of "healthy" vaccine markets.
Other backers of ID2020
include Microsoft and the Rockefeller Foundation, which is also a
significant funder of the WHO.
Verification systems of this size will place the
right of citizens to do basic activities - like traveling, eating at
a restaurant or working their job - in the hands of governments and
potentially employers.
The rights of civilians will be conditional,
dictated by data stored in a massive digital hub that is global in
its sharing abilities.
Not only will domestic governments have
access to the health information of their own citizens under this
system, but an entire global bureaucracy will as well.
Corporate Greed
- A Real
'pandemic'
The patent cliff Big Pharma faces is a steep one,
and the safety barriers that previously kept companies from
descending too far from the top have dwindled away as a result of
the corporate giants' gluttonous consumption of their competition
and industry consolidation over the decades.
With very few traditional drug companies left to
merge with or acquire, the shift towards biotechnology/biologics -
an "unpredictable" type of drug technology designed to target
specific parts of the human anatomy - has begun.
The seemingly
impossible replication of these drugs, their expensive development
and tricky regulatory hurdles in getting their "biosimilar" versions
to market have convinced drug companies that biotech can protect
them from the cavernous patent cliff they must confront.
The dangers
surrounding these drugs, however, create hurdles typical drugs do
not as often have to face in getting to market and earning consumer
trust.
With the WHO now having passed its revised IHR,
and continuing its drafting of the WHO CA+, these policies are
carving out an increasingly likely path for biotech drugs, whether
they are approved through traditional regulatory processes or not.
The One Health model of 'pandemic' preparedness creates an entire
'pandemic' market dedicated to the production of experimental drugs,
brought about through constant biosurveillance of entire populations
and R&D on pathogens with 'pandemic' potential.
Previously, tech
companies such as Palantir have performed this biosurveillance, with
companies like
Google and Oracle taking part in the larger biosurveillance
apparatus.
R&D on dangerous pathogens has been conducted by
Gates-funded NGOs like CEPI - who provided crucial work on the
massively profitable COVID-19 vaccines.
Both the WHO CA+ and the IHR have further
normalized and expanded what was already made the "new normal"
standard during the COVID-19 'pandemic'; specifically, conducting mass biosurveillance to predict and prepare for
'pandemic' outbreaks,
implementing emergency deregulation for experimental drugs to be
distributed in mass while telling people they are "safe
and effective" and conditional mandates that determine one's
human rights based on their vaccination status.
The codification of the right of nations to
demand digital verification and disclosure of people's health
records during a 'pandemic', as well as pressure to supply mass access
of that data through interoperability, permits countries to de
facto take any measure to "slow the spread" of a virus
irrespective of how egregiously it might violate human rights.
The
implementation of the GDHCN would make one's right to bodily
autonomy dependent on their willingness to sacrifice their rights to
travel, and if the technology is repurposed domestically, perhaps
other rights will be removed as well.
The WHO purports that its goal is to "promote
health, keep the world safe and serve the vulnerable", but,
can it
truly do that when implementing international laws that definitively
serve giant corporate interests and diminish the human rights of the
general public?
The financial corruption infecting the organization
has made it subject to the influence of its private stakeholders,
who create policy that enriches Big Pharma.
As the line blurs between the so-called public
and private sector, the greed that drives Big Pharma has reached
completely irrational heights.
The coming biotech 'pandemic' market,
the new heart of our "public health" system, functions on fear,
embodied by the creation of a global biosurveillance system
allegedly meant to prevent ever-increasing 'pandemic's, and
manipulation, disseminated through mandates and information control.
Ironically, while this system is being touted as a form of
'pandemic'
prevention, it potentially incentivizes gain-of-function research
which enables the militaristic weaponization of natural diseases.
Justifying speed over rigor and authority over freedom seem to be
necessary prerequisites for business success in what is becoming the
biotech 'pandemic' market.
Ironically, Big Pharma reached this point
of near-demise as a result of its own desire for wealth and
expansion, and its insistence that medical products must generate
profits over positive health outcomes.
This cultural mindset has led
the world here, in a final faceoff between the globalized corporate
capture of all "public health" institutions, and the truth - the
most potent treatment for this corporate 'pandemic'.
|










